Flavio Noca - flavio.noca@jpl.nasa.gov
Michael Hoenk, Brian Hunt, Dan Choy, Bob Kowalczyk
Microdevices Laboratory
Jet Propulsion Laboratory (JPL)
Pasadena, CA 91109
Jimmy Xu
Division of Engineering
Brown University, Providence, RI 02192
Petros Koumoutsakos, Thomas Werder, Jens Walther
Institute for Computational Sciences
ETH Zurich, Switzerland
Popular version of paper 2aEA2
Presented Tuesday morning, December 5, 2000
ASA/NOISE-CON 2000 Meeting, Newport Beach, CA
The world's most advanced acoustic systems perform incredible feats of detection and targeting, unerringly tracking tiny moving objects from fast-moving ships or other vessels despite severe background noise and clutter. They can detect objects buried in several feet of mud, navigate perfectly in complete darkness, and communicate over long distances in the ocean. While man-made acoustic technologies are better than ever, nature's acoustic systems remain unsurpassed.
At the most basic level, nature's acoustic systems are designed differently from state-of-the-art technology. We rely on ultra-sensitive membrane devices (thin films similar to the skin on a drum) and high-speed computers to detect and analyze sound in a "serial" fashion, or one bit of information at a time. On the other hand, nature uses thousands of tiny rod-like structures (called stereocilia, Figure 1) to detect sound and converts it to nerve impulses for processing in a "parallel" fashion, simultaneously analyzing numerous bits of data. Considering the advantages of stereocilia and neural processing begins to explain why technology has been unable to outperform nature. Moreover, with the help of recent advances in nanotechnology, we hope to create artificial stereocilia to begin learning how we can benefit from nature's example to make better acoustic sensors and systems.
We are presently developing a unique technology that will enable a new class of innovative microsensors and microactuators for real-world applications in gas or liquid environments. The primary product of this research will be the demonstration of acoustic sensing with artificial stereocilia arrays (Figure 2). The miniaturization and directional sensitivity intrinsic to this approach could ultimately lead to revolutionary advances in acoustic detection and signal processing.
Figure 1: Anatomy of the ear. (Top Left) Sketch of the human ear (Alec N. Salt, Washington University). (Top right)
Cross-section through the cochlear canal (R. Eckert et al., "Animal Physiology," 1988). (Bottom Right) Organ of Corti (R. Eckert et al., "Animal Physiology," 1988). (Bottom Left) Stereocilia on inner hair cells. Scale bar: 1 micrometer (J. O. Pickles, "An introduction to the physiology of hearing," 1988).
Stereocilia are rod-like structures (Figure 1) found in the cochlea (inner ear) of all hearing animals. Sound shakes the eardrum, and in turn causes a sliding movement of the endolymphatic fluid (the inner-ear fluid which immerses the cochlea's inner hair cells) and motion of the tectorial membrane, the gel-like covering over a part of the inner ear known as the organs of Corti. This fluid and/or membrane displacement causes the deflection of stereocilia, which is then translated into a nerve signal. The sound of rustling leaves sets the stereocilia in a swinging motion whose amplitude barely exceeds a few atomic diameters! The conversion of acoustic signals into electrical nerve signals in the cochlea of biological organisms relies on the high sensitivity and small scale of stereocilia arrays, which is difficult to duplicate using conventional membrane-based technology for converting between acoustic and electrical signals.
Stereocilia are also present in animals' vestibular systems, which help them maintain balance and orientation. For example, lobsters contain a vestibular organ known as the statocyst which contains stereocilia. Fluid in the statocyst moves the stereocilia relative to the organ's statolith, compacted sand grains that are assimilated by the lobster. Similarly, stereocilia populate fishes' lateral line system, a mechanical-based sensor system for detecting differences in water pressure. They help fish tailor water flows along their body and, presumably, also aid them in spatial identification of preys and predators. Finally, even in non-hearing organisms (hydra, jellyfish, sea anemones), stereocilia may be present as mechanical-based receptors for detecting swimming prey.
The widespread use of stereocilia as acoustic and micro-flow transducers in the biological world represents a powerful incentive to develop acoustic sensor technology based on artificial stereocilia.
A majority of existing acoustic sensors are based on membrane deflection to detect sound. In nature, membranes are generally present as devices for exchanging energy between the acoustic environment and the actual zone where such conversion takes place, typically the cochlea, where the stereocilia are the ultimate performers of this task. Stereocilia are orders of magnitude smaller than membranes, and biological systems use membranes mostly at moderate scales, whereas at scales of microns (millionths of a meter), stereocilia predominate.
The technological difficulty of making nanometer-scale stereocilia geometries has precluded the use of artificial stereocilia in acoustic sensors. Figure 2 shows that biological stereocilia have diameters of the order of tens of nanometers (billionths of a meter) and a large aspect ratio, that is, a large length compared to their small diameters. Conventional MEMS (Micro Electro-Mechanical Systems) fabrication technologies cannot produce objects with diameters this small, and nanometer-scale MEMS is in its infancy. Our approach to this problem is to apply recently developed techniques for fabricating highly uniform arrays of carbon nanotubes that have diameters and aspect ratios comparable to biological stereocilia. The most recently discovered form of pure carbon, carbon nanotubes are rolled-up sheets of carbon atoms arranged in a hexagon pattern.
Figure 2: (Top Left) Highly ordered arrays of
parallel carbon nanotubes. The nanotubes are characterized by a narrow size
distribution, repeating patterns on a large scale and high densities. Ordered nanotubes with
diameters from 10 nanometers to several hundred nanometers and lengths up to 100 micrometers can be
produced. Scale bar: 200 nanometers (J. Li, C. Papadopoulos, J.M. Xu, and M. Moskovits,
Appl. Phys. Lett. Vol 75, 367-369, 1999). (Bottom Left)
Bundle of stereocilia protruding from an inner hair cell of the guinea-pig
cochlea. Scale bar: 500 nanometers (J. O. Pickles, "An introduction to the physiology of hearing," 1988). (Right) Image of a human hair magnified 1000 times (Images of Nature, D. Adams, University of the Western Cape, South Africa, and J. Mayer, Arizona State University). The inset images illustrate the relative size of carbon nanotube arrays, stereocilia, and human hair when placed at the same scale. 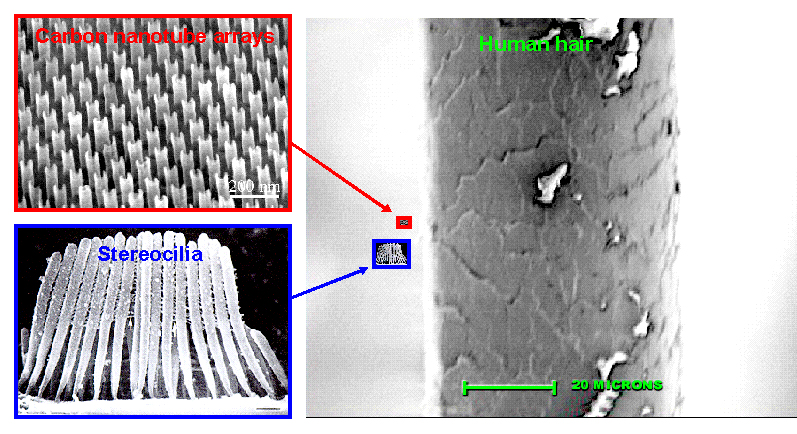
This new nano-fabrication approach places stereocilia-based transducers within reach for the first time. Several unique properties of stereocilia and carbon nanotubes provide motivation for this choice:
While the present bio-inspired technology will benefit the development of a miniature air- or water-coupled acoustic sensor with directional sensitivity, several other applications are envisioned:
Figure 3: The "nanostethoscope" will one day be able
to tap the "sounds of life" by sensing the microflows generated by
biological activity (insets illustrate relative size at the same scale).
( Left)
Microtubule network emanating from the nucleus of a mouse-cell (from G.
Karp "Cell and Molecular Biology," 1999). The microtubules act like a
"freeway network" for the transport of organelles and material across the
cell. The nanostethoscope is envisioned to listen to the buzzing
intracellular activity, and distinguish healthy cells from malignant ones.
(Right) Spermatozoa jiggling around a human ovum (The
Learning Company, 1997). Being able to sense swimming micro-organisms and
biochemical events will help improve water quality and support NASA efforts
in the search for signatures of life on other planets. 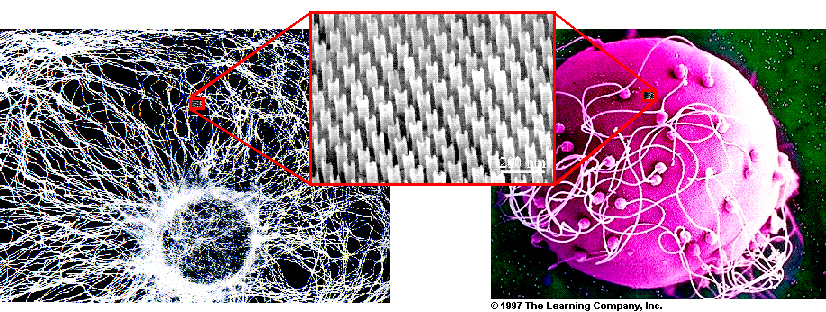
Additional information about the BSICT and CISM programs is available at:
http://cetdp.jpl.nasa.gov/breakthrough.html http://cism.jpl.nasa.gov