151st ASA Meeting, Providence, RI
Knocking On Brain's Door:
Breaking through the barrier using sound
Elisa Konofagou, Ph.D. - ek2191@columbia.edu
James Choi, M.S.- jjc2132@columbia.edu
Department of Biomedical Engineering
Columbia University
351 Engineering Terrace, mail code 8904
1210 Amsterdam Avenue
New York, NY 10027
Web: www.bme.columbia.edu/eekweb
Popular version of paper 1aBB11
Presented Monday morning, June 5, 2006
151st ASA Meeting, Providence, RI
In the late-19th century, a German bacteriologist, Paul Ehrlich, injected a dye into the blood stream of a live animal and observed something very interesting: the entire body of the animal was stained except for its brain. This was an inexplicable phenomenon at the time that remained a mystery throughout Ehrlich's lifetime. What Ehrlich observed was the effect of the blood-brain barrier (BBB), which defines a physical boundary between the blood vessels and the brain tissue surrounding the vessels. One of the differences between the boundary in the brain and in the rest of the organs is that the cells making up the inner lining of the vessels, also called endothelial cells, have smaller gaps between them, forming thus nearly impenetrable links or barriers. The BBB acts as a kind of gatekeeper that determines what molecules can and cannot go through based on various properties such as size. Some larger molecules such as glucose are exceptions to the general rule since they can get actively transported through the barrier. We all have experienced the so-called 'sugar rush' that results from the glucose crossing the BBB and interacting with the central nervous system.
Most of the time, the BBB does not allow molecules to go through. The BBB constitutes therefore the brain's natural defense. It protects the brain from toxic molecules and dangerous substances that typically find it almost impossible to get through this barrier. This is great news for a healthy brain. When the brain develops a disease, however, such as Parkinson's or Alzheimer's, drug molecules have to penetrate that same barrier in order to reach and treat the brain region affected by the disease. Alas, the BBB cannot tell friend from foe. So, drugs, typically involving molecules of size larger than the BBB's threshold, are stopped in their tracks. This has been hindering progress in the drug development and has resulted in the shelving of several potent neurologically-designed drugs.
As a result, an increasingly appealing alternative requires methods for BBB opening prior to injection of the drugs. Opening methods fall under two categories: 1) noninvasive (i.e., through the injection of BBB disrupting molecules) or 2) invasive (i.e., locally through the direct injection of a needle into the brain region where the opening should occur). The former opens the BBB in the entire brain region while the latter may require harmful penetration of unaffected tissue; both therefore incurring undesirable effects.
Only one method can offer both advantages of noninvasive and localized BBB opening: through the use of sound waves. Well, it is not exactly the sound you can hear. It is ultrasound or, sound above the human audible range. The technology is known as Focused Ultrasound Surgery (FUS) or High-Intensity Focused Ultrasound (HIFU). These waves are similar to what is typically used to scan unborn babies in the mother's womb. In the case of FUS, the waves transmitted all have the same intended target and have higher magnitude in order to cause a change of the tissue properties at the target only. To imagine how it works, consider two waves in the ocean. Separately, they have peaks and troughs while traveling through the ocean. If these waves intermix, their peaks (as well as the troughs) combine with each other to create a larger wave. In FUS, an ultrasound source is curved in such a way that the sound waves all combine at a certain point called the focus. In this way, the ultrasound waves are extremely high at the focus, where all the waves synergistically meet, and relatively low everywhere else. Using the same penetration property as diagnostic ultrasound, FUS can act as a method of affecting tissue deep into the body without affecting the surrounding regions. It therefore constitutes a truly noninvasive method. In 2001, Kullervo Hynynen's group at Harvard Medical School was the first to use this technique to open the BBB in rabbits. When the skull of the rabbit was removed and tiny, gas-filled bubbles were injected in the blood stream, FUS waves could cause the BBB to open only at the focus of the ultrasound wave.
In our laboratory, the Ultrasound and Elasticity Imaging Laboratory, at Columbia University we are interested in applying the FUS technique fully noninvasively for the treatment of neurodegenerative diseases such as Alzheimer's. Ulltimately we wish to apply this, in combination with specialized drugs, to patients affected by such disease. The brain region that is typically affected by such diseases is the hippocampus, which is also the main targeted area in our methods. Optimizing this procedure must first be performed in experimental animals. Mice were chosen as the animal model because, due to the advent of transgenic engineering, they are the only animals to have models of neurological diseases such as Alzheimer's, Huntington's, and Parkinson's disease. If mice cannot be used, not many associated drugs can be tested. Our group has developed methods of BBB opening in mice so that a simple ultrasound source can be used to traverse the skull. Figure 1 shows the experimental setup designed for entirely noninvasive BBB opening.
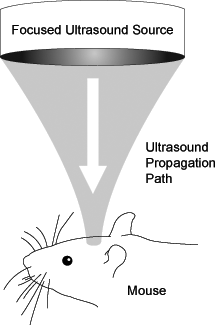
Figure 1. The experimental setup to non-invasively opening the blood-brain barrier in mice. Note how the ultrasound beam targets only a small region of the brain. This allows for precise targeting of where drugs will ultimately be delivered.
Another one of our objectives is to make this technique entirely noninvasive so that the skull and the skin do not have to be removed for the opening to occur. In our experiments, the FUS source was placed a few centimeters away from the mouse's head and targeted through the skull. The skull, which usually poses a problem for ultrasound to travel through, is thin enough in the case of the mouse at the frequency of the beam used for the focus size to be well-formed whether the skull is present (Fig. 2b) or not (Fig. 2a). This method can therefore be rendered completely noninvasive!
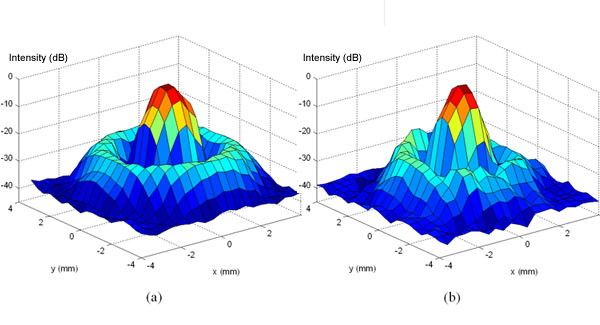
Figure 2. The ultrasound beam shape (a) without and (b) with the mouse skull in place. Note how the general shape of the beam is not significantly changed by the skull.
The effects of the BBB opening in the mouse are studied using high resolution MRI. In order to do this, a molecule that appears bright on the MRI image and does not go through the barrier, due its large size, is used to show which specific region of the barrer has been opened. After opening the BBB using FUS, the mouse brain is scanned after injection of the aforementioned molecule in order to see which part of the brain was "highlighted" indicating where the BBB was open. Consecutive MR images during diffusion of the molecule are typically acquired showing the change of the brain region highlighted, or permeated by the molecule. In our work, we have shown that the entire hippocampus can be highlighted, thereby demonstrating the feasibility of noninvasive treatment of the hippocampus.
Research in this area is ongoing with hopes to ultimately design such a system for human use. In conclusion, should this method be shown feasible for BBB opening in humans, several drug molecules might finally be able to have the desired effect by reaching the brain region they were designed to treat; thereby potentially pushing the frontiers of pharmaceutical science by leaps and bounds.
[ Lay Language Paper Index | Press Room ]
|