Acoustical Society of America
159th Meeting Lay Language Papers
[ Lay Language Paper Index | Press Room ]
Pavlos Anastasiadis - pavlos@hawaii.edu
Stefan Moisyadi
John S. Allen
Department of Mechanical Engineering, University of Hawaii at Manoa
2540 Dole St., Holmes Hall 302
Honolulu, HI 96822
Terry O. Matsunaga
University of Arizona
Tucson, AZ 85724
Popular version of paper 4pBB15
Presented Thursday afternoon, April 22, 2010
159th ASA Meeting, Baltimore, MD
Ultrasound imaging is a well-established modality in clinical diagnosis due to the ability to provide real-time assessment of soft tissue structures and the fact that the levels of invasiveness remain minimal. Diagnostic ultrasound typically operates in the frequency range of 1 MHz to 20 MHz, providing visualization of lesser quality compared to other modalities such as magnetic resonance imaging (MRI), computed tomography (CT) and X-radiation (X-Ray). However, the introduction of ultrasound contrast agents (UCAs) with the initial aim of providing improved image quality has offered new impetus that is transforming ultrasound from a purely diagnostic tool into a therapeutic technique. UCAs conjugated to specific antibodies provide potential molecular imaging capabilities to diagnostic ultrasound. The contrast-enhanced, lipid-encapsulated and gas-filled agents oscillate about their equilibrium radii and produce unique scattering signatures upon ultrasonic irradiation. The conjugation of a targeting ligand such as a monoclonal antibody or peptide to the shell of these echogenic UCA agents provides targeting to specific diseased sites. The use of UCAs for ultrasound-mediated gene delivery opens new avenues to utilize the combination of ultrasound, UCAs and genetic agents for gene therapy. The combination of specificity with the ability to deliver genetic cargo to sites of interest and the fact that ultrasound allows the site and levels of transfection at these sites to be precisely localized and controlled have resulted in the exploitation of this promising technique.
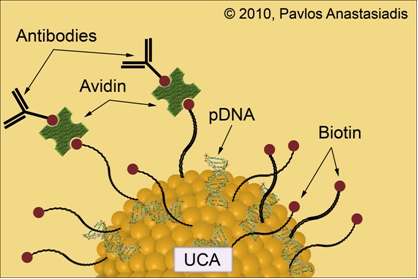
The rapidly increasing understanding of the genetic mechanisms that underlie diseases affecting the life quality of a vast number of individuals is advancing constantly to the extent that genetic manipulations have become part of routine research and practice. Gene therapy is a relatively newly coined term that has resulted as researchers have been striving to tackle diseases at their very genetic origin. The delivery of genetic material into human hosts with the potential of replacing defective genes or inhibiting a preexisting cell malfunction constitutes the objective of these efforts to improve gene therapy and establish it in the clinical setting. The most crucial prerequisite in all forms of genetic manipulation is transfection namely the process of inserting genetic material into cells of a host organism. Transfection has proven an extremely multifaceted procedure and becomes even more complicated due to the fact that DNA is a large and highly charged molecule. Thus, the very nature of DNA prevents its uncomplicated diffusion through the cell membrane demanding for the development of strategies to potentiate the delivery of genetic cargo from the extracellular to the intracellular milieu. Furthermore, there is the distinction between stable transfection, where the transferred gene is incorporated into the genome of a host cell whereas in the case of transient transfection the DNA fragment is expressed although not inserted into the chromosomes.
Currently existing transfection techniques can be divided into two broad categories; viral and non-viral techniques. The very low efficiencies of success encountered with the simple injection of the transgene DNA into host cells has been overcome by the use of deactivated viral particles, which are capable to introduce the genetic material into the recipient cell. Unwanted complications with viruses, such as insertional mutagenesis, host immune response, tumor suppressor gene deactivation, immunogenic properties, inflammation and activation of tumor genes have proven troublesome. Incidents that lead to the death for some of the patients undergoing viral gene therapy have resulted in the suspension of such clinical trials. This has given impetus to approaches for non-viral gene delivery into host organisms, such as transposon-based techniques. Transposons represent gene delivery mechanisms employed by bacteria and eukaryotic systems and are DNA sequences that can move around to different positions within the genome of single cells, a process called transposition. They are also referred to as jumping genes, and are examples of mobile genetic elements. Their mechanism of action has been adapted by scientists to function in circular pieces of DNA called plasmids and insert their cargo into the genome of a host cell that they have been incorporated into. The piggyBac transposase in particular, has been shown to be well-suited for cell transfection and gene therapy approaches due to its flexibility for molecular modification, large cargo capacity and high transposition activity. The single construct, helper independent piggyBac transposase plasmids developed in our laboratories (pmGENIE-3) carry both the piggyBac transposase gene as well as the transposon cargo to be inserted, flanked by terminal repeat element (TRE) sequences. The product of the piggyBac gene binds to the TREs inserting the cargo carried between the repeat elements into the genome of the host cells. Improvements to the helper-independent structure were achieved by developing new plasmids in which the piggyBac transposase gene is rendered inactive after excision of the transposon from the plasmid. As a consequence, potentially negative effects that may develop by the persistence of an active piggyBac transposase gene post-transposition can be eliminated.
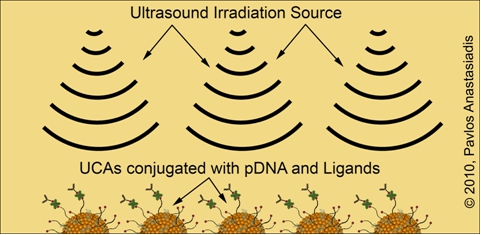
The pmGENIE-3 plasmid is conjugated onto the surface of lipid-encapsulated and gas-filled UCA particles alongside with a combination of ligands that facilitate their binding to specific sites in mouse hosts in vivo. Parallel cell culture experiments are being conducted to allow deepening our understanding about the basic mechanisms that UCAs undergo when within an ultrasonic field of irradiation. Exposure of either tissue in vivo or cells in vitro under well-defined laboratory conditions allows the plasmid to enter through the cell membrane for subsequent expression of reporter genes. Although the mechanisms underlying this enhancement are not well-understood, the main hypothesis is that whenever gas-filled UCA particles are ultrasonically irradiated they start oscillating and cause the formation of transient pores through which the plasmid DNA may gain entrance. Target-site in vivo gene delivery in a precisely orchestrated and controlled manner with increased specificity will remain a major challenge for the years to come. The development of the pmGENIE-3 insertional plasmids in combination with ultrasound-mediated delivery offers new avenues for gene therapy attempts.

