Carhart’s Notch: A window into mechanisms of bone-conducted hearing
Namkeun Kim –kimnk@stanford.edu
Charles R. Steele –chasst@stanford.edu
Sunil Puria –puria@stanford.edu
Stanford University
Department of Mechanical Engineering
496 Lomita Mall, Durand Building
Stanford, CA 94305
Popular version of paper 4aPPa2
Presented Thursday morning, June 6, 2013
ICA 2013 Montreal
Many people are surprised when they hear their recorded voice being played back for them (e.g., on an answering machine), because it sounds different from what they hear when they are speaking aloud. This phenomenon can be explained by the existence of two different hearing pathways, known as ‘air conduction’ (AC) and ‘bone conduction’ (BC). The recorded voice is heard mostly via the AC pathway, whereby sound passes through air from the sound source and into the ear canal, at which point it begins to vibrate the eardrum. These eardrum vibrations are then transmitted to the hearing sensory organ in the inner ear, the ‘cochlea’, through a chain of three tiny bones in the middle ear, known as the ‘malleus’, ‘incus’, and ‘stapes’. On the other hand, a significant part of what we hear of our own voice comes through the BC pathway, whereby the vibrations of the vocal cords cause the bones of the skull to vibrate, leading to stimulation of the cochlea and a resulting hearing sensation. The BC hearing pathway is clinically important for distinguishing between dysfunctions of the middle-ear bones versus the inner-ear nerves or cochlea, due to its ability to stimulate the cochlea whether or not the middle ear is functioning properly. The BC hearing pathway is finding increasing use in modern technology, such as Google Glass, since it can allow audio to be heard without requiring the use of earbuds. In addition, awareness of the BC hearing pathway is important for protecting the cochlea from extremely noisy environments. On an aircraft carrier, for example, many workers complain of serious hearing loss in spite of wearing earplugs or ear mufflers that block sound traveling via the AC hearing pathway. These cases of hearing loss may be the result of too much sound reaching the cochlea via the BC hearing pathway. Therefore, there have been many studies that aim to clarify the mechanisms by which the BC hearing pathway works. According to recent studies, the compressional motion of the outer bony shell of the cochlea and the inertial motion of the middle-ear bones are significant components of the mechanisms of BC hearing. Nevertheless, it is still unclear which of these components dominates the BC hearing pathway for different frequency ranges of input vibration.
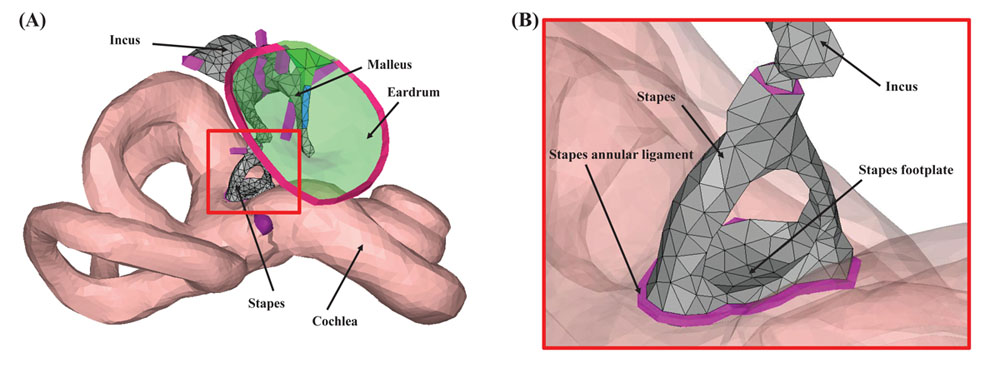
Figure 1. The geometry of the computational model of the human auditory periphery. (A) Middle-ear structures, and the inner ear. (B) Magnified view of the red square in (A). In (B), the eardrum was masked for better visualization of the stapes and stapes annular ligament.
Interestingly, a disease called ‘otosclerosis’, which inhibits the motion of the stapes (the smallest bone in the human body), provides a useful clue for studying which of the BC components is the prevailing one for a given frequency range. The stapes is connected to the cochlea by a soft ligament called the ‘stapes annular ligament’ (SAL). For otosclerosis patients, however, the normally soft SAL becomes ossified such that the stapes can no longer move to transmit sound energy into the cochlea. Because of the ossification of the SAL, the component of BC hearing due to the inertial motion of the middle-ear bones becomes effectively removed.
There have been many studies concerning BC hearing loss in otosclerosis patients. Audiologist Raymond T. Carhart in 1950 was first to show in these patients a 10–20 dB BC hearing loss in the narrow 1–2 kHz range of input frequencies, with no significant hearing loss at lower and higher frequencies, which has since been named ‘Carhart’s notch’ (CN). BC hearing loss for a given otosclerosis patient is defined as the difference in the BC hearing sensitivity between a person with normal hearing and the otosclerosis patient. For example, if the BC hearing loss at 1 kHz is 10 dB, then the otosclerosis patient would need to receive a BC input at 1 kHz that is 10 dB larger than what a person with normal hearing would need in order to perceive the sound at the same level. Most researchers have attributed the CN to the loss of the inertial motion of the middle-ear bones due to the otosclerosis condition. However, this experimental result is somewhat puzzling for the following reason. Since for skull vibration at low frequencies every point of the skull would be shaking together and by the same amount, the middle-ear bones of a normal ear, which are connected to the skull via flexible spring-like ligaments and tendons, would be expected to shake around in their own way relative to the vibrating skull bone. This inertial motion of the middle-ear bones would then be expected to stimulate the cochlea at these low frequencies. On the other hand, since otosclerosis blocks this inertial motion of the middle-ear bones from stimulating the cochlea, one would therefore expect to observe a BC hearing loss at low frequencies for the otosclerotic case. However, this expectation is inconsistent with the experimental data. Why?
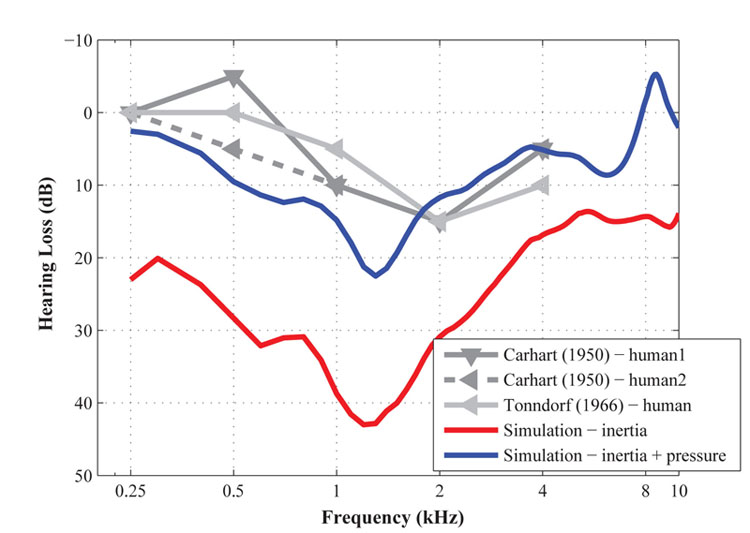
Figure 2. The bone-conducted hearing loss from the otosclerotic condition measured by Carhart (1950) and Tonndorf (1966), and calculated by the current study. The red-solid line and blue-solid lines represent the calculated hearing loss by only the inertia motion of the middle-ear bones, and by combination of the inertia motion and bone compression for BC stimulation, respectively.
In order to answer this question, we built a 3D computational model of the human middle ear and cochlea (Kim et al., 2013), based on realistic anatomical information obtained by scanning the actual structures of a human ear using a device called a micro-CT scanner. To simulate in the model the effects of inertial motion of the middle-ear bones, all of the model’s structures were shaken in a specific direction, and to simulate the effects of bone compression, pressure was applied to the outer bony shell of the cochlea. As the first step, we tested applying only the inertial BC stimulation. Interestingly, as the red-solid line indicates in Figure 2, a notch appeared at 1–2 kHz, but hearing loss at lower frequencies was also observed. This is consistent with what we expected in theory for an inertial BC stimulation, but is inconsistent with the absence of hearing loss observed at the lower frequencies in the clinical data. Therefore, we needed to consider how the addition of a bone-compressional BC stimulus might be able to compensate for this low-frequency hearing loss in the simulation. When as the next step both the inertial and bone-compressional stimuli were applied in the simulation, the bone-compressional input improved BC hearing at lower frequencies shown as the blue-solid line in Figure 2. Given that researchers had previously assumed that bone compression did not play an important role in BC hearing at low frequencies, this finding contributes an improvement to our understanding of the mechanisms of BC hearing. Based on this study, we can conclude that bone compression plays an important role in replicating the low-frequency shape of clinical Carhart’s Notch data from otosclerosis patients, which suggests that the bone-compressional mechanism of the BC pathway is, in fact, important for BC hearing at low frequencies.
Acknowledgments
Research was supported by the National Institute of Deafness and other Communication Disorders (NIDCD) of the National Institutes of Health (NIH) under Award Numbers R01DC05960 and DC07910.
References
(1)
Carhart, R. (1950). “Clinical application of bone conduction audiometry,” Arch. Otolaryn. 51, 798-808.
(2) Tonnforf, J. (1966). “Bone conduction. Studies in experimental animals,” Acta oto-laryng. Suppl. 213, 1-132.
(3) Kim, N.K., Steele, C.R., and Puria, S. (2013). “Effects of the size, location, and shape of a superior-semicircular-canal dehiscence on hearing in humans,” Hear. Res., http://dx.doi.org/10.1016/j.heares.2013.03.008.

