Vasant A. Salgaonkar – salgaonkarv@radonc.ucsf.edu
I-C Hsu
Chris Diederich
Dept. of Radiation Oncology
University of California San Francisco
Viola Rieke
Eugene Ozhinsky
John Kurhanewicz
Dept. of Radiology and Biomedical Imaging
University of California San Francisco
Punit Prakash
Dept. of Electrical and Computer Engineering
Kansas State University
Juan Plata
Dept. of Radiology
Stanford University
Popular version of paper 4pBA2
Presented Thursday afternoon, December 5, 2013
166th ASA Meeting, San Francisco
Introduction
Did you know that ultrasound can be used to treat cancer? Normally associated with pre-natal and fetal imaging, ultrasound can be utilized for incision-less surgery of cancer tumors. Like regular sound waves that we humans hear (< 20 kHz), ultrasound energy is a high-frequency (typically > 500 kHz) mechanical wave. When it travels through a medium like cancer tumors, ultrasound energy gets absorbed and it causes an increase in tumor temperature. Ultrasound energy is used for heat based cancer therapy.
Ultrasound energy can be used to administer hyperthermia treatments where tumors are heated mildly above normal body temperature to about 41 °C or high-temperature (> 60 °C) ablation therapy. Hyperthermia can be used to enhance the efficacy of radiotherapy or chemotherapy. Both radiotherapy and chemotherapy are routinely used to treat cancer. They are limited in total dose delivered, and they can also cause serious side effects to the patient. However when combined with hyperthermia, doses of radiotherapy and chemotherapy get enhanced and patients receive improved treatment benefits with few side effects (Wust 2002; Van der Zee 2002).
Delivering therapeutically significant doses of anti-cancer radiation or drugs to tumors situated deep within the body is always challenging. These demands are encountered in treating prostate cancer which is the second leading cause of cancer related deaths in men and affects 1 in 6 men in the U.S. (Jemal 2011). A hyperthermia boost can improve treatment results, but achieving precise tumor heating while reducing heat exposure to the rectum, urethra, pubic bone and nerve bundles can be complicated.
Study Objective
We explored techniques to adapt and modify ExAblate 2100 Prostate System, a commercial system by InSightec Ltd. approved for magnetic resonance image (MRI) guided prostate ablation, to deliver targeted hyperthermia. We intend to take advantage of the already completed regulatory assessment of this thermal therapy system and fast-track its application in hyperthermia treatment.
Research Strategy
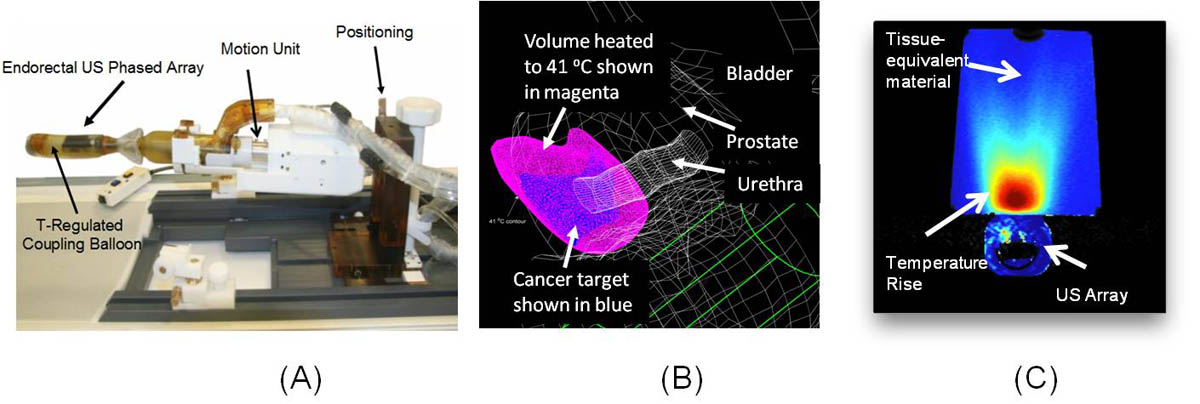
The ExAblate endorectal ultrasound phased comprises a large number of small ultrasound transducers which can be modulated independently to direct ultrasound energy towards the prostate with high accuracy and selectivity from within the rectal cavity (Fig. A). During HIFU ablation, multiple overlapping rice grain size tissue volumes are treated sequentially using highly focused, short-duration (10 - 20 seconds) ultrasound energy bursts to destroy a large cumulative target volume (Napoli 2012).
We have employed mathematical models and experiments in tissue-equivalent phantom materials to determine the feasibility of diffuse, long-duration ultrasound exposures with the ExAblate phased array ablation system to deliver mild temperature hyperthermia to large contiguous tumor volumes in the prostate. We investigated phased array beamforming techniques - methods to modulate power input to individual ultrasound transducers in the phased array - that direct ultrasound energy in patterns that conform to tumor targets. Numerical three-dimensional models of representative prostate cancer volumes and surrounding anatomy were created from MRI images from real patient cases.
Study Results
We were able to simulate focused ultrasound energy patterns that conformed to the shape of small prostate cancer targets (1.5 - 6.0 cm3). More diffused energy patterns with wider energy deposition were simulated to heat larger cancer targets in lateral sections of the prostate (13 - 23 cm3). Mathematical models provide evidence of the ability to heat these target volumes above 41 °C with high selectivity while preventing thermal exposure to surrounding anatomy (Fig. B).
The ExAblate system is designed for image-guided therapy where treatment progress is monitored in real-time using MRI-based temperature measurements. In this scheme, MRI images are processed using customized algorithms which track temperature-induced changes to these images and provide temperature feedback. Phased array beamforming explored during simulation was implemented on the ExAblate prostate array to successfully produce and sustain a 4-12 °C temperature rise in tissue-equivalent material for 15 minute durations (Fig. C).
Conclusions
We were able to demonstrate that the ExAblate system, designed specifically for thermal ablation, can be controlled for delivering continuous hyperthermia in prostate while working within operational constraints.

Fig. #1: (A) ExAblate 2100 ultrasound phased array for high-temperature (> 60 °C) MRI guided prostate ablation (image courtesy InSigtec Ltd), (B) Mathematical model for a focused ultrasound pattern utilized for mild temperature (approximately 41 °C) hyperthermia treatment of small cancer targets (C) Experimental demonstration of heating a tissue-equivalent material with diffused ultrasound pattern using ExAblate 2100 prostate array and temperature measurement using MRI images.
References:
P. Wust, B. Hildebrandt, G. Sreenivasa, B. Rau, J. Gellermann, H. Riess, R. Felix and P. Schlag, "Hyperthermia in combined treatment of cancer," The lancet oncology, 3, 487-497 (2002)
J. Van der Zee, "Heating the patient: a promising approach?," Annals of oncology, 13, 1173-1184 (2002)
A. Jemal, F. Bray, M. M. Center, J. Ferlay, E. Ward and D. Forman, CA: A Cancer Journal for Clinicians 61 (2), 69-90 (2011)
A. Napoli, M. Anzidei, C. De Nunzio, G. Cartocci, V. Panebianco, C. De Dominicis, C. Catalano, F. Petrucci and C. Leonardo, "Real-time Magnetic Resonance guided High-intensity Focused Ultrasound Focal Therapy for Localised Prostate Cancer: Preliminary Experience," European urology, (2012).