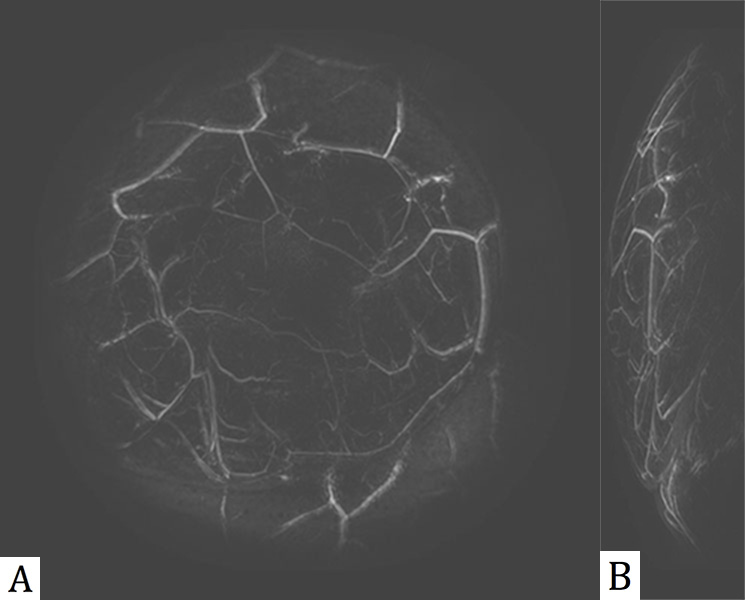
Figure 1. MIP views of a PAM scan showing the vasculature in a normal human breast. A is a coronal view (facing the patient) and B is a lateral (side) view. The blood vessel diameters are from 0.6 to 2 mm.
Robert Kruger –bobkruger@optosonics.com
Richard Lam –dicklam@optosonics.com
Daniel Reinecke –reinecke@optosonics.com
Stephen Del Rio –pdelrio@optosonics.com
OptoSonics, Inc.
108 Straight Road
Oriental, NC 28571
Popular version of paper 1aBA4
Presented Monday morning, May 5, 2014
167th ASA Meeting, Providence
---------------------------------
Today, breast cancer screening relies on traditional two-dimensional X-ray mammograms. However, mammography exhibits a host of problems. The test itself is uncomfortable, requiring the patient to endure painful compression of the breasts. It also exposes the patient to ionizing X-ray radiation that potentially causes tissue damage and limits the number of times the test can be performed. Worst of all, mammograms are difficult to interpret in the case of women with denser breast tissues. Common false positive readings cause extreme anxiety and promote expensive follow-up tests that usually turn out negative.
These follow-up tests rely on techniques such as traditional ultrasound (US) imaging or magnetic resonance imaging (MRI). However, even these approaches are problematic. Ultrasound suffers from a great degree of noise, making the images difficult to interpret. And while MRI provides good, high-resolution 3-D images, it is expensive and requires injecting the patient with a contrast agent, entailing further risk and limiting the repeatability of the test.
To combat these problems, OptoSonics and Canon have partnered to create an entirely new pair of imaging modalities for breast cancer screening. Our approach generates 3-D breast images for cancer screening while the patient lies comfortably face-down on a padded exam table. These new imaging techniques are painless and quick, require no breast compression, no exposure to X-ray radiation, and no injection of contrast agents. Furthermore, the tests can be repeated as needed with no danger to the patient.
Both of these new techniques, High Definition Ultrasound (HD-US) and Photoacoustic Mammography (PAM) are based on a single hemispherical array of detectors that capture both photoacoustic signals and reflected ultrasound signals from the breast. This novel arrangement of detectors, coupled with a spiral scan of the detector array, collects data from the breast tissue at over one million different angles in just under two minutes. By mathematically analyzing the data over all of these different projections, we form extremely high-resolution 3-D images of the interior of the breast.
The PAM images are formed via the photoacoustic effect, first discovered by Alexander Graham Bell. One of the patient's breasts rests in a plastic cup partially filled with water. This cup is suspended over the hemispherical detector array that is scanned in a spiral pattern beneath the breast cup. At the bottom of the detector array, a laser pulses a near-infrared light beam into the breast. The laser is tuned to a wavelength where hemoglobin absorbs the light. This absorbed light causes the molecules to generate a photo-excited sound wave in the ultrasound frequency range. These ultrasonic waves then propagate through the breast to the detector array where the signals are captured and analyzed.
PAM images capture information up to 5 cm deep in the breast, at a resolution of less than 1 mm. This provides an excellent view of the breast vasculature, which is modified by new feeding vessels when cancer is present. Figure 1 shows coronal and lateral maximum intensity projection (MIP) views from a PAM scan of a normal human breast, and Figure 2 is a movie of a rotating 3D PAM breast image.

Figure 1. MIP views of a PAM scan showing the vasculature in a normal human breast. A is a coronal view (facing the patient) and B is a lateral (side) view. The blood vessel diameters are from 0.6 to 2 mm.
Figure 2. Animation showing a rotating 3D PAM breast image.
While vasculature changes are linked to some breast cancers, other types of cancers are indicated by micro-calcifications that are typically detected via X-rays. Our preliminary work has demonstrated detection of micro-calcifications as small as 200 microns in phantoms using our hemispherical array approach (Figure 3). Today we are proposing HD-US as a new imaging modality complementary to the PAM approach.
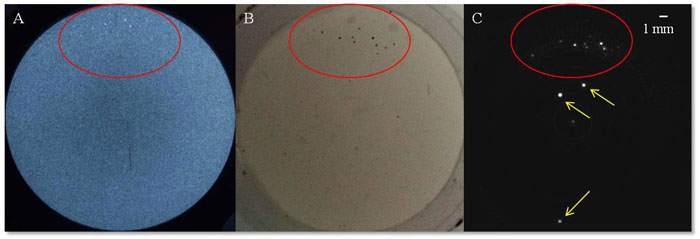
Figure 3. Views of a phantom containing micro-calcifications (< 200 microns in size) using (A) traditional mammography, (B) a light photograph, and (C) a HD-US backscattered view.
Our HD-US uses the PAM hemispherical detector array, but adds instrumentation to send out ultrasonic waves and then receive the backscattered waves for each detector in the array. During a spiral scan, each element is sequentially pulsed and data captured on the same element. In this manner, the backscattered ultrasonic waves are captured and analyzed without any correlation between elements that is typical of other medical linear or curved planar sampling techniques.
This configuration will produce direct 3-D maps of ultrasound reflectivity of breast tissues with higher signal-to-noise, reduced speckle and greater spatial resolution compared to traditional ultrasound images (Figure 4). And as the same detector array is used while the patient is in position, the HD-US images will be co-registered with the PAM images, producing 3-D images of soft tissue, micro-calcifications and vasculature.
Figure 4. 3-D rotated views of a phantom containing micro-calcifications using (A) traditional B-mode ultrasound using a linear array and (B) HD-US using the hemispherical array.
OptoSonics and Canon are currently integrating the HD-US mode with an existing PAM device. Meanwhile, PAM clinical trials begin this summer at the University of North Carolina Department of Mammography and at Kyoto University in Japan. HD-US and PAM are poised for a revolution in breast cancer screening.
References
1. R.A. Kruger, R.B. Lam, D.R. Reinecke, S.P. Del Rio and R.P. Doyle, "Photoacoustic angiography of the breast", Med. Phys. 37 (11), 2010.
2. R.A. Kruger, C.M. Kuzmiak, R.B. Lam, D.R. Reinecke, S.P. Del Rio and D. Steed, "Dedicated 3D photoacoustic breast imaging", Med. Phys. 40 (11), 2013.
Acknowledgements
The authors gratefully acknowledge support from the National Institutes of Health (HHS Grants 1-R01-CA160850 and 1-R43-CA183113), and the ongoing support and partnership with Canon, Inc.