Acoustical Society of America
ICA/ASA '98 Lay Language Papers
Fish Love Songs
Andrew H. Bass1,2- ahb3@cornell.edu
Deana Bodnar1
1Section of Neurobiology and Behavior
Cornell University
Ithaca, NY 14853
2UC Bodega Marine Laboratory
Bodega Bay, CA 94923
Popular version of paper 4aAB1
Presented Thursday morning June 25, 1998
ICA/ASA '98, Seattle, WA
ACOUSTIC COMMUNICATION SIGNALS
Each summer, male plainfin midshipman fish migrate from deep offshore sites into the intertidal zone along the western coast of North America (ref. 1). There, they excavate den-like shelters beneath rocks to form nests. From those nests, males emit their chant-like, mate call known as a "hum" (Fig. 1A). Hums are low pitch sounds which often remind us of the sound of a drone of bees or of an outboard motor. In technical terms these sounds are periodic signals with a sinusoidal-like waveform. Every periodic signal can be dissected into a set of sinusoidal waveforms that have a fundamental frequency and a number of harmonics that are multiples of the fundmental. The fundamental frequency of a midshipman hum characterizes its pitch or periodic repitition rate, while the harmonics characterize its timbre or quality.
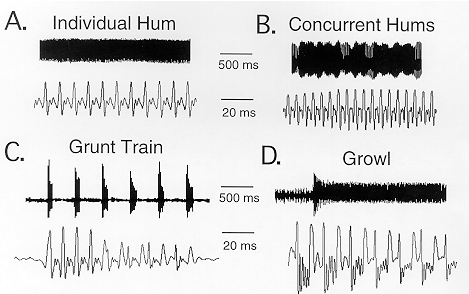
Nesting males, now known as "type I" males, will generate a single hum continuously for minutes on end, sometimes for over an hour. Using underwater loudspeakers, computer-synthesized acoustic signals can be played back to individual fish and their behavioral responses observed (5). Experiments such as these have shown that hum-like signals and not other vocalizations (see below) or white noise, attract females with eggs to a speaker. Together with earlier observational studies of spawning behavior (4), the results conclusively show that the hum functions at least as a male mate call. Interestingly, some type I males are also attracted to a single hum, although their responsiveness does not compare to that of a female. It is likely that the hum serves some other function for these individuals. For example, a type I male, as perhaps also females, may simply utilize the hum as a "beacon" that more generally indicates a locale for suitable nest sites.
Midshipman have a second group of smaller, "type II" males that do not acoustically court females but rather sneak into a nest while females are there with a type I male (4). Type I and II males are easily distinguished on the basis of features such as body size and shape. As with type I males, some type II males are also attracted to a hum. Perhaps type II males also utilize a hum to find suitable nest sites or more specifically to localize nests that contain gravid females.
Besides hums, type I males produce "grunt trains" which are a rapid succession of brief duration signals (time scale of msec) at intervals of about 400 msec (Fig. 1B); they function in an agonistic context when an egg-guarding type I male is challenged by other males. "Growls" are somewhat intermediate in duration (time scale of seconds to minutes; (Fig. 1D) and are also produced in agonistic contexts.
Acoustic "beats" are a fourth class of signals that result from the overlap of the hums of neighboring type I males (Fig. 1C). Nesting males occur in clusters so that two nests are often side by side. Concurrent hums sum to produce acoustic "beats". An egg-bearing female will directly approach the signal emitted from one underwater speaker when two hum-like tones with differences in fundamental frequencies comparable to those between type I males are presented independently through two underwater speakers.
In sum, vocal communication plays an important role in the social behavior of midshipman fish. But what part does the nervous system play in the performance of these tasks?
TEMPORAL FEATURES OF CALL PRODUCTION
One goal of auditory neuroscience has been to identify how brain cells or neurons determine the physical attributes of acoustic signals. The vocal motor system of teleost fishes such as midshipman has provided a powerful model for such studies because the physical attributes of their acoustic signals can be understood in terms of the electrical activity of the nervous system. Take for example the fundamental frequency of a hum which characterizes the periodic repitition rate for each cycle of the vocalization. Recordings of the activity of individual neurons in the brain have shown that the hum's fundamental frequency is directly established by the periodic nerve impulse (action potentials) activity of a cluster of neurons (pacemaker nucleus). The discharge frequency of a vocal pacemaker circuit describes the rate at which the brain generates single action potentials (2). Thus, if the fundamental frequency of a hum is 100 Hz, then single vocal pacemaker neurons are producing action potentials at 100 Hz. This, in turn, leads to the simultaneous contraction at a rate of 100 Hz of a pair of sound-generating muscles attached to the walls of the fish's swimbladder, which leads to the 100 Hz fundamental frequency of the hum. What is critical here is that the stereotyped appearance of the time-varying acoustic waveform is established by the rhythmicity of the brain's vocal pacemaker.
TEMPORAL CODING OF ACOUSTIC SIGNALS
The simplicity of midshipman vocalizations, especially the sinusoidal-like hum, permit the generation of computer-synthesized sounds that readily mimic naturalistic stimuli and can be used in studies to reliably assess how the brain transforms species-typical sounds into a neural code of action potentials.
There are many ways to characterize the responsivity of neurons to acoustic signals. One traditional method is to establish what is known as tuning curves that use spike rate, the number of action potentials produced per unit time, as a measure of the frequency or spectral sensitivity of a neuron. Our studies of the encoding of vocalizations in the brain have focused on a region known as the midbrain which is an important center for auditory processing in the brains of all vertebrates including humans. As defined by spike rate, the majority of midbrain neurons in midshipman have broad tuning properties although they have "best frequencies" centered near the fundamental frequencies of their vocal signals (3).
More recently, we have focused our efforts on trying to understand how the time-varying properties of action potentials, for example the intervals between successive spikes or the synchronization of spikes to specific portions of a single cycle of the acoustic waveform, generate a "temporal" code of sounds. These methods have now revealed how midshipman encode the difference frequencies generated by the overlap of the hums of neighboring males. We have studied the encoding of the separate and combined fundamental frequencies of two pure tones that form simple beat-like signals that mimic natural beats (3). These studies demonstrate a temporal coding of beats as defined by the vector strength of synchronization, a measure of the stereotypy with which action potentials occur at a particular phase in a single cycle of the acoustic waveform (also known as phase-locking). While midbrain neurons display relatively low synchronization to the individual tonal components of a beat, they show high synchronization to the the difference in the fundamental frequencies of the component tones which describes the rate of amplitude modulation of a beat. Hence, one explanation for how individual midshipman fish choose one of two concurrent hum-like signals that form an acoustic beat is that they have neurons with temporal coding properties that permit the segregation and discrmination of the fine temporal structure of single and concurrent hums.
Together, our studies provide an exquisite example of how the brain
both generates and detects rhythmic, time-varying acoustic waveforms;
they also show how sensory and motor systems have co-evolved to permit
effective acoustic communication.
ACKNOWLEDGMENTS
The authors' work is supported by funds from NSF and NIH.
REFERENCES
1. Bass, A.H.,
2. Bass, A.H. and Baker, R.,
3. Bodnar, D.A and Bass, A.H.,
4. Brantley, R. K. and Bass, A. H.
5. McKibben, J.R. and Bass, A. H.
Fig. 1. Acoustic signals of midshipman fish. The temporal waveform of
each signal is shown on two different time scales. Shown are
representative examples of a hum (A), concurrent hums
(beats;