Acoustical Society of America
ICA/ASA '98 Lay Language Papers
Targeted Acoustic Contrast Agents:
New Opportunities for Ultrasound in
Medical Diagnosis and Therapy
Gregory M. Lanza - greg@soundlab.wustl.edu
Division of Cardiology
Box 8020
Barnes-Jewish Hospital
Washington University
216 S. Kingshighway Blvd.
St. Louis, MO, 63110
Popular version of paper 4pBV3
Presented Thursday afternoon June 25, 1998
ICA/ASA '98, Seattle, WA
For 30 years, medical professionals have sought the "magic bullet" which would allow sensitive detection of disease and facilitate localized administration of potent chemotherapeutic agents without systemic toxicities. Ligand targeted ultrasonic contrast agents have the potential to improve diagnosis of disease and to treat those maladies with targeted local delivery of therapeutics. A ligand directed, lipid encapsulated perfluorocarbon emulsion particle (250 nm diameter) contrast system has been developed and demonstrated both in vitro and in vivo . The agent is a weak ultrasonic scatterer except when bound to a target where the aggregated particles enhance the overall acoustic brightness of the tissue surfaces. The magnitude of enhancement has been tentatively explained by a simple mathematical model. The contrast agent has a long, one hour circulatory half-life which facilitates successful systemic targeting. The small particle size of these emulsions affords access to tiny capillary beds and vascular endothelial disruptions such as those post- angioplasty. These studies suggest perfluorocarbon contrast agents can target molecular epitopes which are key to the development of cancers and clots and create a new generation of opportunities for ultrasound in medical diagnosis and therapy.
Often ultrasonic contrast differences between healthy and pathological tissues are subtle and difficult to detect. New ultrasonic blood pool agents, primarily microbubble formulations, have improved delineation of cardiac anatomy by demarcating vascular spaces. However, these agent can not specifically differentiate abnormal from normal tissues except as revealed by altered blood flow patterns. We have recently developed a novel, targeted contrast system 1 based upon emulsion technology. The particle is a 250 nm, lipid encapsulated, emulsion which takes advantage of natural avidin (eqq white protein) - biotin (vitamin) interactions to couple antibodies in vivo to the contrast particle.
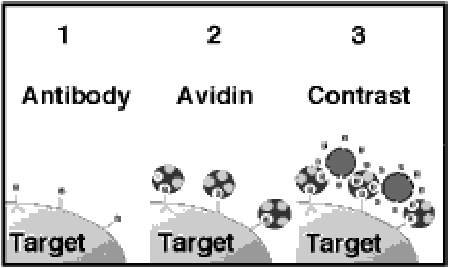
Figure 1. Three-step targeted contrast system
The ligand-directed system utilizes three steps. A biotin conjugated monoclonal antibody (biotinylated antibody) is administered first and binds the target tissue. Avidin is given next which binds to and cross-links the biotinylated antibody on the targeted tissue surface. The biotinylated emulsion is administered thirdly and binds to the previously given avidin through remaining, unoccupied biotin binding sites. The apposition and concentration of contrast particles over the surface of the tissue increases the ultrasonicreflectivity of the tissue as imaged with routine 2D echocardiographic images. The low inherent echogenicity of the the circulating contrast particles maximizes the overall detectability of the contrast effects.
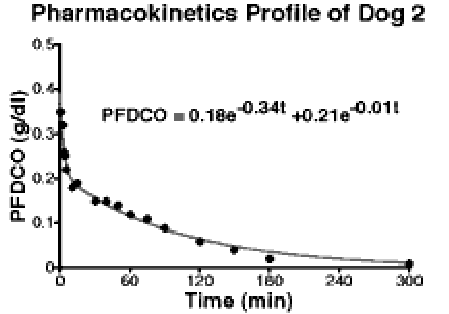
Figure 2. Typical pharmacokinetic profile of targeted contrast system
To date, several investigators have utilized biotinylated antibodies and labeled avidins or biotins to target and detect pathologic tissues but the improvements have been poor regardless of the imaging modality2. Attempts to enhance these applications with particles, typically liposomes, have worked well in vitro but generally failed in vivo due to inadequate circulating half-life or impaired targeting characteristics 3. The pharmacokinetic profiles of the emulsion contrast system following intravenous bolus injection was studied in three canines. In each animal, intravenous access was established in the brachial vein and emulsion (10 cc) was administered as a bolus. Venous blood samples were drawn and analyzed in duplicate. A model was fit to the data and a half-life (t1/2 ) of 64 3 minutes was determined (Figure 2).
The efficacy of the contrast agent to target carotid arterial thrombi pre-exposed to anti-fibrin antibody 4 and avidin in 9 mongrel dogs following intravenous injection (the limiting step for particulates) was evaluated. As noted in Figure 3, the targeted contrast emulsion was able to circulate, localize and clearly enhance the acoustic image of the clots obtained with a commercially available echocardiography system (7.5 Mhz).
Acoustic microscopy studies were employed to quantify the increase in ultrasonic reflectivity when the contrast system bound to substrates of varying acoustic character . The targeted contrast system was bound to nitrocellulose membranes, whole blood clots and acellular fibrin clots which increased the average acoustic reflectivity by 7.9 1.1, 8.6 0.9 and 36.0 2.6 dB, respectively. Moreover, using reasonable assumptions, first-order approximations of reflectivity enhancement predicted using a simple linear acoustics transmission line model, 5, 8 and 29 dB, respectively. This model suggests that the emulsion particles bind to the surface and in the aggregate create a layer effect which enhances the acoustic reflectivity of the surfaces, analogous to the reflection of light by a mirror.
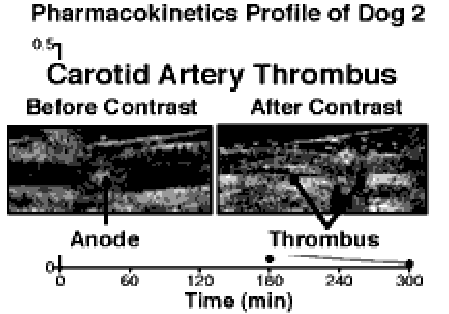
Figure 3. Increased acoustic reflectivity of canine arterial thrombus following targeted contrast agent.
The targeted contrast agent also can delineate the induction of protein expression associated with pathologic processes by penetrating through microscopic disruptions of porcine carotid endothelial linings to specifically localize "tissue factor" elaborated within the wall of balloon-injured arteries. Accordingly, tissue factor is an important initiating factor in arterial clot formation in atherosclerosis, leading to artery occlusion, and has been associated with arterial closure after angioplasty [Oltrona, 1997 #1854]. Both carotid arteries of three pigs (20kg) were injured by angioplasty balloon. The carotids were treated locally with either targeted contrast agent employing an antibody to tissue factor or to a control contrast agent. Intravascular ultrasound (20 Mhz) was used to image the arteries before and after exposure to the emulsions. The acoustic reflectivity of the subintima and tunica media at the site of balloon- induced injury was markedly enhanced in comparison to the control contrast arteries. (P<0.05) (Figure 4.) Immunohistochemical staining confirmed the presence of increased tissue factor at the sites of acoustic enhancement.
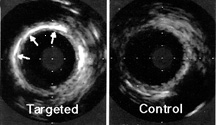
Figure 4. Enhancement of tissue-factor paotangioplasty with targeted emulsion contrast.
These studies suggest that targeted contrast agents can enhance the acoustic reflectivity of abnormal tissues accessible from the circulatory bed. The agents will improve the diagnostic sensitivity and specificity of medical ultrasound studies. Moreover, their particulate nature is expected to facilitate the local delivery of therapeutic drugs and genes for the treatment of many diseases, particularly solid tumor cancer.
References
1. G. Lanza, K. Wallace, M. Scott, W. Cachetis, D. Abendschein, D. Christy, A. Sharkey, J. Miller, P. Gaffney, and S. Wickline. "A novel site-targeted ultrasonic contrast agent with broad biomedical application," Ciculation 94, 3334-3340 (1996).
2. G. Paganelli, P. Magnani, F. Zito, E. Villa, F. Sudati, L. Lopalco, C. Rossetti, M. Malcovati, F. Chiolcrio, E. Seccamani, and F. F., "Three-step monoclonal antibody tumor targeting in carcinoembryonic antigen-positive patients." Cancer Res. 51, 5960-5966 (1991).
3. I. Ogihara-Umeda, T. Sasaki, H. Toyama, K. Oda, M. Senda, and H. Nishigori, "Rapid tumor imaging by active background reduction usingbiotin-bearing liposomes and avidin.," Cancer Res. 54, 463-7 (1994).
4. S. Raul and P. Gaffney, "Evaluation of fibrin binding profile of two antitibrin monoclonal antibodies," Thromb Haemost 76, 56-64 (1996).