Acoustical Society of America
136th Meeting Lay Language Papers
[ Lay Language Paper Index | Press Room ]
Ultrasonic Periodontal Probe: A painless way to monitor gum disease
Prof. Mark Hinders- hinders@as.wm.edu
and John Companion
Department of Applied Science
College of William & Mary
Williamsburg, VA 23187
Contact Information During ASA Meeting:
vmail: (757) 221-1519
Popular version of paper 4pBB7
Presented Thursday afternoon, October 15, 1998
136th ASA Meeting, Norfolk, VA
Periodontal disease (gum disease) is one of the most pervasive dental diseases in older adults. It involves the loss of connective tissue attachment with subsequent destruction of tooth-supporting bone, leading to loss of teeth. At present there are no reliable clinical indicators of periodontal disease activity and the best available diagnostic aid, conventional periodontal probing, is only a retrospective analysis of attachment already lost. Subtraction radiography (x-rays) may be of value in detecting small changes in alveolar bone mineralization but does not evaluate periodontal ligament attachment. In addition, changes in bone have been shown to lag behind connective tissue loss by several months. Serial radiography also subjects the patient to increasing amounts of ionizing radiation. A method for detecting small increments of periodontal ligament breakdown would permit earlier diagnosis and intervention with less costly and time consuming therapies. Moreover, there is evidence that ``disease active'' sites respond positively to therapy but that quiescent or stable sites do not change or lose attachment so a more sensitive diagnostic probe would permit site-specific identification of attachment loss. This could direct treatment toward areas that are actively breaking down, and eliminate over treatment for sites that are stable. The development of an ultrasonic alternative to conventional periodontal probing promises a much better understanding of the pathogenesis of periodontal disease and will provide the clinician with a non-invasive method for measuring periodontal status without the often-reported discomfort of conventional periodontal probing.
The main use of ultrasound in dentistry is for scaling of teeth and internal shaping of teeth which is in contrast to other areas of medicine where diagnostic ultrasonography is a standard clinical imaging technology. Ultrasound imaging has been recognized by leading authorities as having the best potential for non-invasive periodontal disease evaluation and initial attempts at using ultrasound for intra-oral diagnosis have shown promise despite difficult technology problems. Our exploratory development program has now produced an intra-oral probe small enough to be practical with an ultrasound beam projection area close enough in size to the width of the periodontal space to give the optimal coupling and small enough to inspect the area between the teeth, while still delivering sufficient signal strength and depth of penetration to image the periodontal space.
We have developed a non-invasive ultrasound technique to detect, image, and map the upper boundary of the periodontal ligament and its variation over time as an indicator of the presence of periodontal disease. One of the key technical obstacles we overcame was developing an ultrasonic probe that would be small enough to be useful, but yet transmit and receive sufficient signal strength. The space occupied by the periodontal ligament is normally on the order of 0.5 mm wide, located between the outer surface of the tooth root and the inner surface of the bone forming the socket in which the tooth resides. The coronal elevation of the periodontal ligament is normally approximately 1 mm below the surface of the junctional epithelium which abuts the tooth surface and forms the sulcus below the gingival margin. In order to probe these structures ultrasonically, a narrow beam of ultrasonic energy is projected down between the tooth and bone from a transducer which is manually scanned along the gingival margin, as shown in Figure 1.
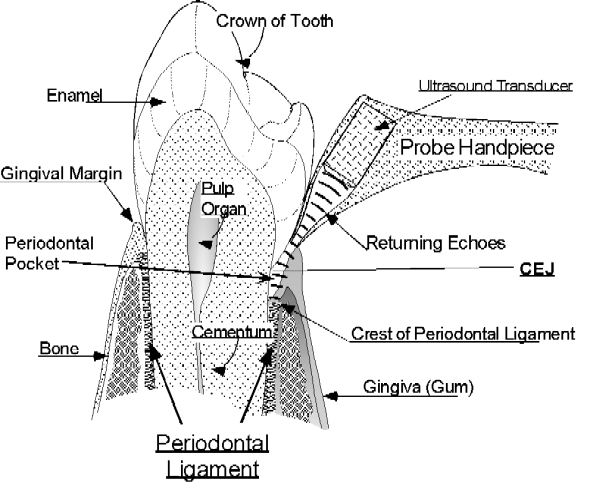
Figure 1. Ultrasonic periodontal probe with probe tip at the gingival margin and ultrasound projected into the periodontal pocket. The echoes returning from the crest of the periodontal ligament are shown.
The transducer is mounted at the base of a dual taper, convergent-divergent coupler, in order to provide an acoustically tapered interface with a throat area on the order of 0.5mm. This constitutes an active area reduction from the transducer element to the aperture of 20-1. Such a reduction is mandated by the geometry and the very small window afforded by the gingival margin. An added virtue of attaining this small a tip size is the ability of the ultrasonic probe to examine the area between the teeth, which is where the problem of periodontal disease is most likely to occur. Figure 2 shows how the ultrasound transducer is mounted in the probe tip shell, which is also incorporates a slight flow of water to ensure good coupling of the ultrasonic energy to the tissues. The probe tip can be mounted in a hand piece that is light in weight and a convenient size for clinical use. The couplant water can come either from a suspended IV-type sterile bag or plumbed from the dental chair. All of the specialized electronics are commercially available as plug in computer boards and are operated via software that runs on the type of pentium class PC's that are becoming common in the dental office.
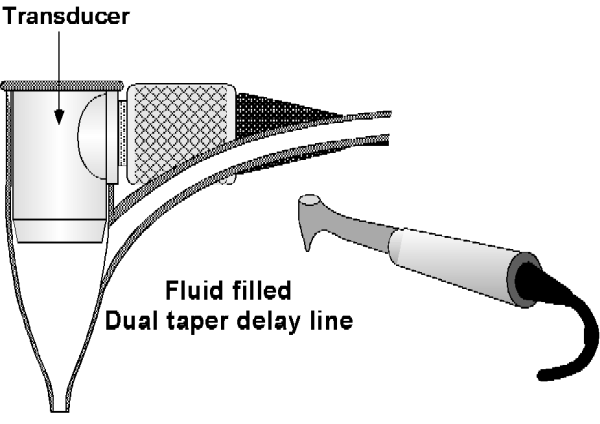
Figure 2. The hand piece contains a probe tip which is small enough to permit scanning of the area between teeth. The ultrasonic transducer is mounted in a probe tip shell (a fluid-filled dual taper delay line) which has a throat diameter small enough to project the ultrasonic beam into the narrow space between the tooth and bone.
The focused ultrasonic beam is transmitted into the pocket in the same orientation as a manual probe is inserted and the probe is then moved along the gingival margin, so the two dimensional graphical output corresponds to that one gets from ``walking the sulcus'' with a manual probe. Ultrasound gives more information, however, because secondary echoes are recorded from tissue features at various depths. It appears likely that the technique will also be able to provide information on the condition of the gingival tissue and the quality and extent of the epithelial bond to the tooth surface. This may provide valuable data to aid the clinician in the diagnosis and treatment charting of the disease.
The authors thank Cpt. A. Charles Richardson, DDS of the Naval Dental School for providing the samples used in this work, and for many helpful discussions. The support and assistance of Drs. Eric Madaras and Joe Heyman of NASA Langley Research Center are also gratefully acknowledged. This work was supported by NDS and NASA Langley, with the concept patented by: Companion, JA (1998) Differential Measurement Periodontal Structures Mapping System, US Patent #5,755,571, assigned to NASA.