Neil Owen - nowen@apl.washington.edu
Michael R. Bailey
James Hossack
Larry Crum
Center for Industrial and Medical Ultrasound
Applied Physics Laboratory
University of Washington
1013 NE 40th St.
Seattle, WA 98105
Yongmin Kim
Departments of Bioengineering and Electrical Engineering
University of Washington
Box 357962
Harris Hydraulics Laboratory, Room 309E
Seattle, WA 98195-7962
Popular version of paper 2pBB1
Presented Tuesday afternoon, November 11, 2003
146th ASA meeting, Austin, TX
Injuries characterized by blunt trauma frequently involve the bleeding of internal organs. Two events that might cause this condition are car accidents or battlefield combat, and there are many others. Internal bleeds can be life threatening because the victim can lose blood very quickly while there may be no obvious external injuries. In addition, hemostasis (the medical term for stopping bleeding) for some internal organs can be difficult to achieve with traditional surgical techniques.
It has been shown during intra-operative experiments that acoustic energy (ultrasound) can be used to stop bleeds. In these intra-operative experiments, the internal organs are exposed the acoustic energy is applied directly to the injury. Energy is delivered to with an ultrasound transducer that is used to convert an electronic signal into acoustic waves, which then propagates to the intended treatment site. The transducer is curved and works like a lens to focus the acoustic energy within a volume that is similar in size to a grain of rice. The ultrasound intensity within the focus is high enough to bring human tissue to a temperature of 70 degrees Celsius or higher in less than one second. With high temperatures in such a small, well-defined region, it is possible to apply the ultrasound energy selectively to cauterize the injury and achieve hemostasis, with little or no damage to the surrounding tissue. Therapeutic ultrasound used in this manner is aptly called high-intensity focused ultrasound (HIFU).
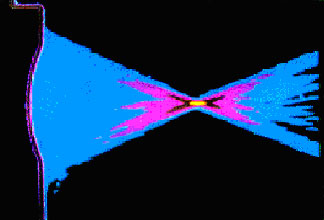
A false color image of the measured pattern of a focused
transducer.
Note the small and well-defined focal zone which enables precise
internal acoustic energy delivery and internal cauterization.
In addition to the intra-operative treatments mentioned above, HIFU can also be used transcutaneously, meaning the ultrasound can pass through the skin and other intervening tissue between the injury and the transducer. Intervening tissue is not damaged because of the focusing effect described above, where the high temperatures are only present within the focus. This makes it possible to perform hemostasis procedures without any incisions, and therefore with much less risk of subsequent infection. However, transcutaneous treatments require that a medical imaging modality be used to locate the injury and monitor the treatment.
An ultrasound imaging device can be used to guide hemostasis procedures. A prominent advantage for using ultrasound instead of another imaging modality is portability. Many portable ultrasound imagers are similar in size to a laptop computer, while other imaging devices, such as a magnetic resonance imaging (MRI) machine, can be the size of a small car. Another advantage of ultrasound is that the image information is available in real-time, meaning the delay between new images is so short that it is not perceivable to the human eye. However, using ultrasound for imaging and for hemostasis treatments is difficult because energy from the high intensity source can interfere with the imaging device.
To build a new image, or frame, of ultrasound information, the ultrasound imager
must perform transmit, receive, and computational functions. Building one frame
can be thought of as an imaging cycle. For transmit, several bursts of ultrasound
are transmitted to the patient through an imaging transducer. Next, the imaging
transducer receives the echoes from the transmit pulses. To finish, electronics
within the ultrasound imager combine the received echoes and perform computations
to build an image that is displayed on the screen.
When the HIFU transducer is transmitting, the echoes that would be received
by the imaging transducer are distorted and the overall image building process
is disrupted. Part, or all, of the display screen on the ultrasound imager is
obscured by noise when this happens. However, in order to monitor the treatment
process, the treated area must remain visible on the display screen of the imager.
It is possible to control the location of the noise on the screen through precise
timing between the HIFU excitation and the imaging cycle. The HIFU must be turned
on and off at very specific intervals in relation to the imaging cycle to make
sure the treatment area remains visible during a hemostasis treatment. Typically,
such synchronization has been achieved using multiple instruments tuned to work
for a specific setup of the ultrasound transducer and the HIFU.
In this paper, we present a new synchronization system that solves the problem of interference between the ultrasound imaging device and the HIFU source. Where other systems use expensive instruments that are dedicated to a particular imager, we use the HIFU transducer as a receiver that can detect the transmit pulses from the imaging transducer. As the imaging ultrasound propagates through the tissue, the echoes are received by the HIFU transducer. These echoes are used to precisely control the HIFU excitation in relation to the imaging cycle. Even if settings on the imager are changed (depth or imaging modality), the system can remain synchronized because it responds to the transmit portion of the imaging cycle.
The result is a HIFU excitation that is exactly synchronized with the imaging cycle of the ultrasound imager. From the user's perspective, the display screen of the ultrasound imager looks normal and clear from interference while diagnosing an internal bleed. Once the bleed is located and the user decides to begin treatment, only part of the screen, roughly 30 %, is obscured with noise caused by interference. The center of the image, which is used to monitor the treatment, remains clear because the location of the noise can be controlled. Plus, because the system responds to the imager, it will remain synchronized when the user changes settings on the imager. This is important because it makes the system easier to use and allows the user to concentrate on the patient.
Other synchronization systems can provide the same function, but their cost
is much higher. Either the imaging transducers must be modified or detailed
knowledge of a particular imager is required. Our synchronization system is
instrumental for transcutaneous hemostasis research because it allows a HIFU
transducer to be used with almost any ultrasound imager. This significantly
reduces the cost and, because the system responds to the imager, it offers adaptive
features that other methods do not provide.