


Bones in the light of ultrasound
Pascal Laugier, laugier@lip.bhdc.jussieu.fr
University Pierre et Marie Curie-Paris 6, Laboratoire d’Imagerie Paramétrique, UMR CNRS 7623
15 rue de l’Ecole de Médecine, F- 75006 Paris France
Popular version of paper 4pBBa1, "Present state and future trends in ultrasonic characterization of bone"
Presented Thursday Afternoon, July 3, 2008 in ROOM 352B.
Ask yourself what makes the strength of a building such as the Eiffel tower, i.e., its ability to withstand bending and shearing forces of the wind. The quantity of scrap used to build it? The intrinsic strength of each iron beam? The structure (i.e., size, shape, orientation of the beams, overall shape of the building)? All these factors contribute to the strength would answer the engineer.
The Eiffel tower was surprisingly inspired by the work in early 1850s of the anatomist Hermann von Meyer on the anatomy of the femur (thighbone). Like engineers who control the integrity and the strength of buildings (towers, bridges, …), physicians scrutinize the strength of our bones, specifically to detect fragile bones and identify subjects at fracture risk and in need for treatment.
Fragile bone are commonly (but not exclusively!) encountered in a disease called osteoporosis (or porous bone) characterized by a decrease in bone mass and structural and material deterioration of bone, leading to increased susceptibility to fractures of the hip, spine and wrist (i n simpler terms, osteoporosis is a condition in which the bones become weak and can break from a minor fall or, in serious cases, from a simple action such as a sneeze) . Osteoporosis is most common in women after menopause, but may also develop in men, and may occur in anyone in the presence of particular hormonal disorders and other chronic diseases or as a result of medications , for example long-term corticotherapy. Osteoporosis may significantly affect life expectancy (one-year mortality rates after hip fracture range from 15 to 20 percent ) and quality of life. Osteoporosis is a major public health threat with extremely high costs to health care systems. Approximately one in two women and one in four men over age 50 will have an osteoporosis related fracture in their remaining lifetime. The costs to governments measure in the billions of dollars annually, and these numbers are destined to increase, with as many as 6.3 million hip fractures predicted annually, around the world, by 2050. Clinicians and researchers alike are emphasizing the importance of early detection of osteoporosis and fracture prevention.
Bone quantitative ultrasound
Today, radiological (X-ray) measured bone mass serves as a surrogate for bone fragility, but fails to take into account other important aspects like material strength or structure. Mechanical waves such as ultrasound are intrinsically suited to probe mechanical properties and may perhaps have the best chances of all modalities to yield non-invasively an improved estimation of bone fragility combined with advantages like lack of ionizing radiation and cost-effectiveness. Ultrasound cannot be heard but pass through the human tissue, including bone, and thus can be used to see into the body. And like for X-rays, ultrasound cast shadows due to attenuation of the tissue. Their velocity and degree of penetration depends on the matter they are traveling through. For example, the more porous bones are, the better the penetration is (less attenuation) and the lower the velocity is (slower wave). Consequently, bone tissue often is characterized in terms of ultrasound velocity and attenuation.
Although the clinical potential of ultrasound for the investigation of bone fragility was recognized as early as in the 1950s where an ultrasound method was described for monitoring fracture healing [1] , ultrasound was used little to investigate bone properties until the 1990s. The reason that ultrasound were not used before this date was because of immature technology and poor understanding of the interaction mechanisms between ultrasound and bone. In 1984 Chris Langton et al. took a step forward by discovering that the transmission of ultrasound through the heel could discriminate osteoporotic from non-osteoporotic women [2] . He demonstrated that the heel of osteoporotic patients could transmit ultrasound waves better than that of an age-matched normal subjects. Since then many advances have been achieved by our group and others and a variety of different sophisticated technologies capable of measuring different skeletal sites such as the heel, fingers, wrist or leg have been introduced and evaluated. The evidence that ultrasound is a valid (radiation free and inexpensive) method for fracture risk assessment is first class. In recent years, several devices received FDA approval that further opened the door to clinical acceptance and use. Bone ultrasound technology, termed QUS (Quantitative Ultrasound), gained a place in the armamentarium of modalities used to assess the skeleton.
While the concept of measuring attenuation and velocity of ultrasound in bone has changed little since its inception, technology has evolved. Quantitative ultrasound imaging of the skeleton, introduced by our group in 1996 was first applied to image the heel (Fig.1) [3] .
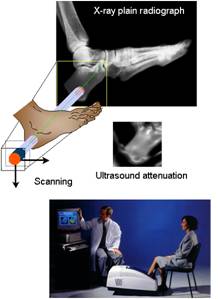
Fig. 1 : In vivo ultrasound attenuation image of the heel. A pair of transducers are placed on opposite side of the heel. The image is obtained by scanning the heel and by recording the wave that passes through the heel at each scan point. The ultrasound image, compared to the X-ray plain radiography, shows high quality details. Evidence that QUS at the heel can be used to predict fracture risk is first class. Several heel devices received FDA approval.
The image that is viewed results from the ultrasound passing through the skeletal site being inspected and interacting with a piezoelectric receiving transducer that transforms the transmitted wave into an electric signal. Local values of the attenuation or of the wave speed, computed from the measured electric signal, are coded in grey (or color) level at the pixel in the image. As in plain radiography, the attenuation image formed is a "negative image" since brighter areas on the image indicate where lower levels of transmitted ultrasound reached the receiver. In other words, the lighter, brighter areas represent thicker sections or more dense sections of the inspected skeleton site. Today, we are able to generate real-time parametric images through the use of two-dimensional arrays of transducers [4] . Technological advances have provided clinicians with smaller, lighter, and very portable equipment such as that inexpensive device operated with four AAA batteries [5] .
An important limitation of QUS today is their limited access to peripheral skeletal sites only. One of the most significant recent technological advances is the a new QUS scanner developed by Barkmann et al. for direct assessment of skeletal properties at the proximal femur (hip) (Fig.2) [6] .
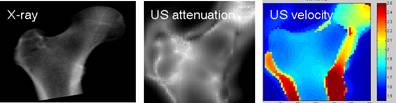
Fig. 2 : In vitro QUS images of attenuation (middle) and sound velocity (rigth) of the femur compared to the X-ray bone mass image (left). The ligther areas on the ultrasound attenuation image represent areas of higher attenuation, i.e., thicker or more dense regions of the femur. These brighter regions on the ultrasound attenuation image corresponds also to brighter regions on the X-ray image. Recent works have shown that ultrasound measurements could predict femoral bone mass with a reasonable accuracy. Preliminary in vivo results have been recently published [6]. The left panel shows an image of the sound velocity. Interestingly, high velocity values are found at the boundary of the femur. We hypothesize that these high values correspond to the propagation of guided waves in the dense cortical shell that surrounds the femur. Measuring these guided waves would provide invaluable information on the cortical shell properties.
For X-ray based techniques measurements directly at the main osteoporotic fracture sites have proved to be superior to measurements in the peripheral skeleton. It is reasonable to also expect better hip fracture risk prediction for QUS assessment at the proximal femur compared to the heel. However, the complexity of the anatomy makes measurements at this site quite challenging.
More recently the emphasis of innovative QUS basic research has shifted towards cortical long bone measurements, such as the tibia (leg) or the radius (forearm). Like tube or pipelines inspected by non destructive ultrasonic testing methods, long bones can be probed by ultrasound waves produced in response to an impact (the ultrasound impulse) transmitted by a source to the bone through the soft tissue. The method has been adapted by our group to reduce artifacts caused by soft tissue [7] (Fig. 3).
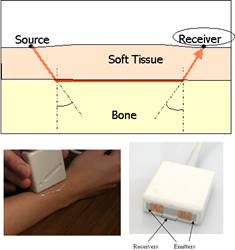
Fig. 3 : Illustration of the technique implemented to measure long cortical bones. The measurement configuration consists in emitters and receivers placed on the skin on the same side of the inspected skeletal site, and the velocity is determined for an ultrasonic wave transmitted in the direction of the bone axis. Our group has developed a probe utilizing several ultrasonic transmitters to allow specific measurement sequences, the so-called bidirectional axial transmission technique, aiming at overcoming the effect soft tissue. In the bidirectional axial transmission approach, an ultrasonic pulse is transmitted along the bone surface in two opposite directions from two sources placed at both ends of a unique group of receivers. A simple combination of the time delays derived from waves propagating in opposite directions efficiently corrects automatically for soft tissue and for probe inclination with respect to bone surface.
Waves propagate along the bone and their velocity, related to bone properties, is computed from measurements performed at distance from the source. Interestingly, long bones support the propagation of different kind of waves, such as surface or guided waves which contain relevant information on microstructural and material properties. Judicious choice of propagation modes over a suitable frequency range can be achieved and subsequent measurements of their velocities can reflect distinct aspects of bone quality [8, 9] , hoping that they would appropriately reflect the bone quality status at the main fracture sites (e.g., hip or spine) and its changes associated with disease or treatment.
QUS techniques could find widespread clinical use to predict bone fragility not only in osteoporotic patients, but also in a wider context of bone diseases in female, male and pediatric populations. For example, preliminary studies suggest that this technique may be a useful method of assessing changes in bone health in preterm infants for whom X-ray technologies is unsuitable for such settings. An ultrasound wearable system for remote monitoring of the healing process in fractured long bones has also been reported [10] .
Models
QUS techniques and implementations have been introduced into clinical practice despite the fact that the interpretation of QUS data is hampered by the structural complexity of bone. Interaction mechanisms between ultrasound and bone are still poorly understood . Modeling can be seen as a major need in order to drive future experiments, to optimize measurements, to integrate multiscale knowledge, to relate QUS variables to relevant bone biomechanical properties. U ltrasound propagation through bone is complex. It may involves different waves types, each with its own propagation characteristics. Ultrasound may propagate along curved paths, thus complicating the retrieval of the velocity. An accurate interpretation of ultrasound measurement results requires first a detailed understanding of ultrasound propagation with clear identification of the different waves and their exact propagation paths. The complex structure of bone significantly complicates the task of solving equations, though.
Recently developed computer simulation tools offer a fertile alternative to intractable theoretical formulations. Computer simulation will likely have its greatest impact by allowing the researcher to visualize the propagation of ultrasound trough the very complex three-dimensional bone structures, and by providing insight into the interaction mechanisms between ultrasound and bone. Simulators and computers may well become theprimary tool for investigators to answer questions such as : how is the wave transmitted through the bone, what is the path followed by the wave? How does it interact with bone? What kind of wave is propagating ? Computer simulations, illustrated on Figure 4, have been applied to the problem of transmission through pieces of spongy bone (such as that found in the femur at the hip), and along or across long cortical bones such as the radius [11-13] . In every case the computer simulations provided valuable insight into the properties (e.g., nature and pathway) of the propagating waves.
Computer simulation therefore resembles experiments in a virtual laboratory with independent control over each bone parameter. Virtual scenarios of osteoporosis for instance can be easily implemented, and used to form a comprehensive understanding of bone ultrasonic properties and their relation to bone biomechanical competence [14] , help validate or refute theoretical approaches, and probe new experimental configurations.
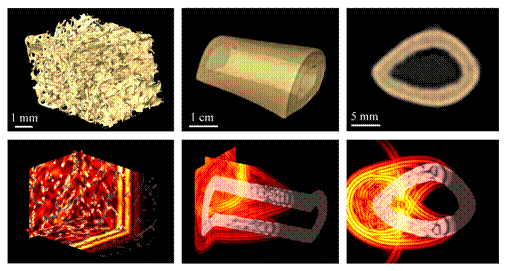
Fig. 4: The figure illustrates Finite-Difference Time-Domain (FDTD) simulations of wave propagation through bone specimens immersed in water. Such simulations are currently being performed on numerical models of bone by coupling a FDTD code with numerical three-dimensional (3-D) bone structures reconstructed from X-ray computed tomography data acquisitions. Image courtesy of Emmanuel Bossy and Pascal Laugier. Emmanuel Bossy is with the Laboratoire Photons et Matière, ESPCI/CNRS, Paris ; Pascal Laugier is with Université Pierre et Marie Curie, Paris France. This figure has made the front cover of the June issue of IEEE Trans Ultrason Ferreoelc Freq Contr (vol. 55, n°6).
Typical snapshots of wave propagation (bottom) are presented for three distinct applications (top) : 3-D spongious femur microstructure (left panel), 3-D human radius diaphysis (middle panel) and 2-D transverse cross-section of a human radius (right panel). Several logarithmic color scales were used to represent absolute values of the displacement velocity wave field, and to allow for a qualitative visualization of waves of different amplitudes combined with transparency effects to visualize bone. The possibility of applying the 3-D FDTD approach to actual bone structures provides a valuable tool to study transmission of ultrasound waves through cancellous and cortical bone and to elucidate the interaction mechanisms between ultrasound and bone structures. A 3-D snapshot of a 1-MHz quasi-plane wave propagating through a trabecular bone microstructure is shown on the left panel. A coherent ballistic plane wave is clearly seen, followed by a spatially incoherent scattered wave. Waves transmitted axially along the long axis of a human diaphysis are illustrated on the middle panel. Their nature are determined by the wavelength-to-cortical thickness ratio. Of particular interest are the leaky waves that can be detected with transducers placed on the skin using the so-called axial transmission technique. The right panel shows the transmission of a 1-MHz quasi-plane wave in a transverse cortical experimental configuration. Circumferential guided waves propagating in the cortex are shown together with a wave front directly transmitted through the medullary canal.
Conclusion
Although the methodology for assessing bone properties using ultrasound is much less developed to date than with x-rays, t he potential of ultrasound extends far beyond the currently available techniques and is largely unexploited. M any new areas of investigation are in preliminary stages, though. Most active research is carried out in QUS to develop new measurement modes, access to the central skeleton (hip), exploit multiple propagation modes or extend the frequency range of the measurements. All these new developments should result in new QUS variables and systems able to provide information on material or structural properties other than density, and ultimately on osteoporotic fracture risk.
Bibliography
[1] I. M. Siegel, G. T. Anast, and T. Fieflds, "The determination of fracture healing by measurement of sound velocity across the fracture site.," Surg Gynecol Obstet, vol. 107, pp. 327-32, 1958.
[2] C. M. Langton, S. B. Palmer, and S. W. Porter, "The measurement of broadband ultrasonic attenuation in cancellous bone," Eng Med, vol. 13, pp. 89-91, 1984.
[3] P. Laugier, B. Fournier, and G. Berger, "Ultrasound parametric imaging of the calcaneus: in vivo results with a new device," Calcif Tissue Int, vol. 58, pp. 326-31, 1996.
[4] M. A. Gomez, M. Defontaine, B. Giraudeau, E. Camus, L. Colin, P. Laugier, and F. Patat, "In vivo performance of a matrix-based quantitative ultrasound imaging device dedicated to calcaneus investigation," Ultrasound Med Biol, vol. 28, pp. 1285-93., 2002.
[5] J. J. Kaufman, G. Luoc, D. Conroyd, W. A. Johnsone, R. L. Altmane, and R. S. Siffert, "New ultrasound system for bone assessment," presented at Medical Imaging, San Diego, 2004.
[6] R. Barkmann, P. Laugier, U. Moser, S. Dencks, M. Klausner, F. Padilla, G. Haiat, M. Heller, and C.-C. Glüer, "In vivo measurements of ultrasound transmission through the human proximal femur " Ultrasound Med Biol, 2008.
[7] E. Bossy, M. Talmant, M. Defontaine, F. Patat, and P. Laugier, "Bidirectional axial transmission can improve accuracy and precision of ultrasonic velocity measurement in cortical bone: a validation on test materials," IEEE Trans Ultrason Ferroelectr Freq Control, vol. 51, pp. 71-9, 2004.
[8] P. Laugier, C. Baron, and M. Talmant, "Quantitative ultrasound for cortical bone characterization," Osteoporosis Int, vol. 19, In press.
[9] A. Tatarinov, N. Sarvazyan, and A. Sarvazyan, "Use of multiple acoustic wave modes for assessment of long bones: model study," Ultrasonics, vol. 43, pp. 672-80, 2005.
[10] V. C. Protopappas, D. A. Baga, D. I. Fotiadis, A. C. Likas, A. A. Papachristos, and K. N. Malizos, "An ultrasound wearable system for the monitoring and acceleration of fracture healing in long bones," IEEE Trans Biomed Eng, vol. 52, pp. 1597-608, 2005.
[11] E. Bossy, F. Padilla, F. Peyrin, and P. Laugier, "Three-dimensional simulation of ultrasound propagation through trabecular bone structures measured by synchrotron microtomography," Phys Med Biol, vol. 50, pp. 545-56, 2005.
[12] E. Bossy, M. Talmant, and P. Laugier, "Three-dimensional simulations of ultrasonic axial transmission velocity measurement on cortical bone models," J Acoust Soc Am, vol. 115, pp. 2314-24., 2004.
[13] P. Moilanen, M. Talmant, P. H. Nicholson, S. Cheng, J. Timonen, and P. Laugier, "Ultrasonically determined thickness of long cortical bones: Three-dimensional simulations of in vitro experiments," J Acoust Soc Am, vol. 122, pp. 2439-45, 2007.
[14] G. Haïat, F. Padilla, F. Peyrin, and P. Laugier, "Variation of ultrasonic parameters with microstructure and material properties of trabecular bone: a 3D model simulation," J Bone Miner Res, vol. 22, pp. 665-74, 2007.