Seek
and Destroy Tumors with Ultrasound
Elisa E. Konofagou - ek2191@columbia.edu
Caroline Maleke - cm2243@bcolumbia.edu
Gary Yi Hou - yh2367@columbia.edu
Jianwen Luo - jl2767@columbia.edu
Department of Biomedical Engineering,
New York,
Breast cancer is the most frequently diagnosed cancer (excluding skin cancer), and remains the No. 2 cancer killer (after lung cancer) in U.S. women. The incidence of breast cancer has been rising steadily since 1960. Currently, one woman in seven will develop breast cancer in her lifetime. The American Cancer Society estimates that 212,920 new cases of invasive breast cancer will be diagnosed in 2009, and 43,300 patients (i.e., 20.3%) will die from the disease. The evaluation of breast masses in men and women is similar, typically involving mammography, MRI and ultrasound.
Surgical intervention is warranted in most cases. However, as tumors are being detected at earlier stages, new methods for less invasive and more focal treatments are warranted. There are several options for breast cancer treatment such as chemotherapy, hormone therapy, radiation therapy, Radio-Frequency (RF) ablation and High-intensity focused ultrasound (HIFU). The latter is the only, truly noninvasive and non-ionizing treatment method that can be applied extracorporeally and, in principle, at very low cost. However, its translation to the clinic has been hindered in part by costly monitoring albeit high image quality techniques.
In this talk, we present a technique that localizes and targets tumors for ablation in the breast based on their distinct hardness. After localization, the same technique monitors the ablation procedure, also based on the hardness variation as the tumor is burnt.
Our technique of Harmonic Motion Imaging (HMI) aims at regionally vibrating the breast, before, during and after treatment and obtaining a map of its hardness at each step. The principle is based on palpation that has been used over several centuries to localize abnormalities at the physicians fingertips. Palpation is still used extensively in a clinical setting to determine the size, hardness, and location of a suspicious mass for the detection of tumors in organs such as the breast, thyroid, prostate and liver. Conventional imaging techniques such as computed tomography (CT), magnetic resonance imaging (MRI) and mammography can detect tumors based on their structural characteristics; however, these modalities do not provide the information elicited upon palpation, i.e., the hardness of the region probed. Accurate depiction of tissue elasticity for accurately and efficiently detecting tumors is therefore warranted.
On the other hand, in clinical practice, surgery aims at effectively resecting the tumors. Surgery itself may pose significant risks to patients. Some of the known risks associated with surgical removal of solid tumors include complications from anesthesia, infections, immune suppression, and long recovery periods. Therefore, if a non- invasive technique can properly treat the same tissue volume, the outcome of disease-free survival should be at least equivalent. The development of minimally invasive methods as alternatives to surgery for localized tumor destruction has been of interest in the field of tumor treatment. Such techniques include radio-frequency ablation (RFA), cryoablation, laser ablation, and High-Intensity Focused Ultrasound (HIFU).
Treatment with HIFU is often considered to be a promising technology within minimal, or non-invasive, therapy segments of medical research. Recently, the potential of HIFU as a non-invasive surgical tool has been demonstrated in several clinical applications for tumor treatment of the prostate, liver, kidney, pancreas, breast, and uterine fibroids. Magnetic Resonance Imaging (MRI) and ultrasound imaging-based methods have been tailored for HIFU treatment guidance in order to accurately identify the target volume.
HMI is a novel imaging technique and an ultrasound based technique for tumor detection, classification, and treatment. It uses amplitude-modulated (AM) waves to generate an oscillatory force (i.e., vibration) deep inside the organ. This vibration acts like a palpation to sense the tissue geometry and mechanics (such as size, shape, stiffness, or location) through tissue deformation. After HMI identifies the location and size of the tumor, HMI can be used to treat the detected tumor by delivering a high level of thermal energy up to 80C to kill cancer cells.
HMI has been studied using a simulation model and tested on tissue-mimicking gel and has also been demonstrated to accurately map 17 post-surgical breast specimens (i.e., normal, benign, and malignant tissues) and reliably differentiate between normal, benign and malignant human breast tumors based on their distinct hardness when probed with HMI.
The integration of imaging and treatment into one method is known as the HMI for Focused Ultrasound (HMIFU) system (Fig. 1). HMIFU system can be used as an image guidance tool for visualization of the targeted tissue (e.g. tumor), and generation and monitoring of the relative tissue stiffness changes during heating. HMIFU is applied in 12 porcine liver specimens and used to treat breast tumors in 11 live mice. The results show that the HMIFU system could follow the tissue hardness change during heating and reliably indicate the onset of coagulation necrosis so that the treatment procedure can be performed in a precise and optimal treatment time (i.e., thermal dose) and controlled lesion size.
In conclusion, we demonstrate that HMI can combine imaging and therapeutics for cancer treatment into a single, all-ultrasound, fully-integrated system. HMI may thus constitute a non-ionizing, cost-efficient and reliable method for tumor localization, charactarization and targeting for ablation, as well as simultaneously monitor in real time, tumor thermal treatment; all in a unit portable and wheelable into any hospital unit, whether as an outpatient or inpatient procedure.
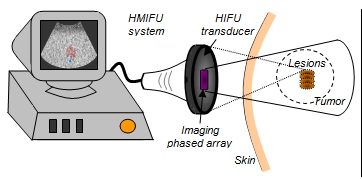
Figure 1. In vivo HMIFU showing the HMI image on the ultrasound system screen (left) during HIFU with several lesions formed in the detected tumor.