Pocket-Sized Therapeutic and Diagnostic Ultrasound
Devices: From the Lab Bench to Clinical Trials
George K. Lewis
Jr. -
Zachary
R. Schulz and William L. Olbricht
Department
of Biomedical Engineering
Cornell
University
Ithaca,
NY 14850
Jason
A. Spector and Peter Henderson
Department
of Surgery
Weill
Cornell Medical College
New
York, NY 10065
Susan
C. Pannullo
Department
of Neurosurgery
Weill
Cornell Medical College
New
York, NY 10065
M.
Cary Reid
Department
of Geriatrics & Gerontology
Weill
Cornell Medical College
New
York, NY 10065
Ralph
Ortiz
Pain
Management Specialists
Dryden,
NY 13053
Steven
A. Gelber
OB/GYN
Associates
Department
of Obstetrics & Gynecology
Cayuga
Medical Hospital
Ithaca,
NY 14850
George
K. Lewis Sr.
Transducer
Engineering Inc.
Andover,
MA 01810
Popular
version of paper 1pBB14
Presented
Monday afternoon, April 19, 2010
159th
ASA Meeting, Baltimore, MD
Translational
research is the hallmark of biomedical engineering with a positioned outcome of
improving the quality and duration of life for mankind. Our team of engineers
and clinicians seeks to solve problems that touch close to home and affect
millions of people every year. Our drive is to quickly innovate and prototype
ultrasound-based solutions and place them into clinical hands for evaluation,
preliminary testing and clinical-feedback as quickly as possible. This rapid, iterative
approach to our research is possible because we possess the facility and talent
to develop every piece of an ultrasound based system in our biomedical
acoustics laboratory. From the onset of every project our team tackles,
clinically inspired motivation drives engineering design innovation, while our
collaborations drive technology translation.
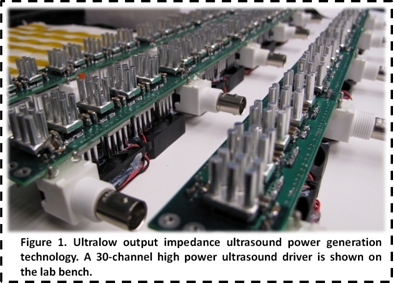
Our
team of ultrasound engineers from the Department of Biomedical Engineering at Cornell
University developed a platform ultrasound technology in 2007 that reduces the
cost and size of ultrasound devices by orders of magnitude (Figure 1). The
principles behind the technology are to reduce the output impedance of the
ultrasound generator and the input impedance of the ultrasound transducer to
zero, to create zero resistance to energy flow and optimize electrical power
transfer for battery powered ultrasound devices. This pioneering approach of
zero output and input impedance pushes the efficiency of ultrasound systems,
and provides ultrasound power in a pocket-sized form. Since our technologys
inception, motivations from physicians have driven ultrasonic innovations to
improve drug delivery in glioblastoma brain cancer therapy, develop non-invasive
varicose vein treatment systems, apply ultrasound over extended periods as a
pharmaceutical-free approach to pain management, and improve fetal heart rate
monitoring to allow easy and consistent measurements during labor.
Ultrasound-assisted
Brain Cancer Therapy (In Vivo
Preclinical Studies): Scientists in our laboratory have developed and commenced
testing of a new ultrasound-based drug delivery system for pre and post-resection
treatment of high-grade malignant gliomas. Surgery and adjuvant radiation are
standard treatments for these malignancies. However, invasive malignant cells
migrate into surrounding healthy tissue and, as a consequence, are not all removed
in surgery, leading to tumor recurrence, usually close to the site of the
original tumor. Convection enhanced delivery (CED) has emerged as a leading
investigational delivery technique for the treatment of several disorders,
including glioblastoma, which presents an especially
poor prognosis for patients. CED bypasses the blood-brain barrier by infusing
compounds through a needle or microcatheter directly
into the brain parenchyma or brain tumor. The clinical trials of CED show mixed
results and suggest that the outcome of therapy depends strongly on the extent
of penetration of drug into the brain, which is determined by infusion
velocity, and the relative rates of convection and elimination during CED. In
collaboration with Drs. Susan Pannullo and George Lewis
Sr. of the Department of Neurosurgery at Weill Cornell Medical College (WCMC)
and Transducer Engineering Incm., respectively, we have
developed ultrasound-assisted convection enhanced drug delivery technology
(UCED) to improve the penetration and spatial control of pharmaceuticals in the
brain (Figure 2).
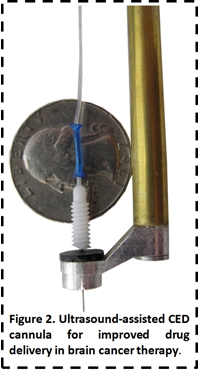
We
have developed in vitro and in vivo models of UCED brain tumor
treatments and have shown that combining ultrasound with traditional CED
improves the penetration and distribution of tracer molecules by 4-6 times in vivo. This work involves both the basic
science of transport mechanisms as well as the translational science of scaling
the UCED brain cancer therapy into a large animal glioblastoma model at Cornell
Veterinary Medical Center. If successful, we will transition the technology to
human treatment in the next few years.
Non-invasive
High Intensity Focused Ultrasound Varicose Vein Treatment (In Vivo Preclinical Studies): Varicose veins affect more than 30
million people in the United States each year. They cause emotional distress
and discomfort for patients and, if left untreated, can progress to deep venous
thrombosis, skin ulceration, limb loss or death. Clinicians perform more than
150,000 varicose vein treatments in the U.S. each year, using methods such as
vein stripping, sclerotherapy, and endovenous laser and RF treatment, which together comprise
a $450MM market. Because these methods
are invasive, they incur added costs in training, equipment, facilities, and
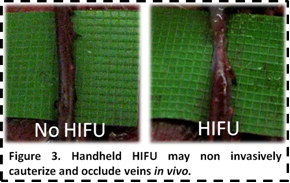
staff. In collaboration with Drs. Jason Spector and
Peter Henderson from the Department of Surgery at WCMC, we have developed and
tested the first battery-powered handheld HIFU system to non-invasively
cauterize and occlude varicose veins (Figure 3).
By
focusing ultrasound energy to a sharp point with the handheld HIFU system, we
are able to successfully ablate and occlude veins without damaging surrounding
tissue. The handheld device has gone through multiple design iterations with
our team, and we have tested the device in both ex vivo and in vivo
platforms. We will soon be incorporating low-cost ultrasound image guidance
into the system and begin testing on large animal porcine models. The
technology has potential to be utilized similar to a Bovie
Pen for tissue cauterization in a range of clinical applications.
Wearable
Ultrasound Pain Therapy Patch (Clinical Studies): Ultrasound therapy for pain
and healing has been approved by the U.S. FDA and has been in use around the
globe for the last 60 years. Diathermy, tissue-regeneration, pain relief and
rehabilitation applications of ultrasound are primarily driven by the positive results
obtained during treatments. Traditionally, ultrasound-mediated treatment has
been limited to short and confined periods of 15-25 min at acoustic intensities
from 1-4W/cm2 over a course of weeks to months. Over the past
decade, research has increasingly focused on lower-intensity ultrasound
(30-1000 mW/cm2) delivered over extended
1-8hr periods. Recent studies using low-intensity ultrasound have demonstrated
successful muscle rehabilitation, and tendon and facture healing resulting in
pain relief. It is believed that using a lower-intensity ultrasonic treatment
rgime over extended treatment periods works better with the bodys natural
healing process and minimizes acoustic insult as compared to traditional higher
intensity, short-term treatments. Working with Drs. Cary Reid and Ralph Ortiz
from WCMC and Pain Management Specialists respectively, and the Clinical
Translational Science Center, we are testing the first iPod sized ultrasound
therapy device on a range of disorders including tennis elbow, arthritis,
fibromyalgia, tendon and ligament tears, muscle spasms, and joint inflammation
(Figure 4).
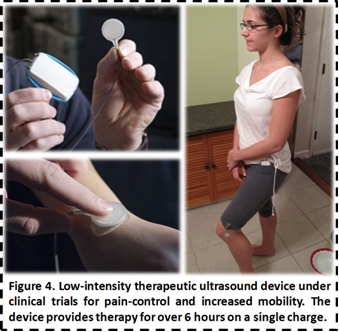
We
are currently conducting multiple pilot studies in an effort to reduce pain,
increase mobility, and improve quality of life for multiple patients suffering
from chronic pain issues. We have further initiated the process of conducting a
50-100 patient clinical trial using the ultrasound device on osteoarthritis of
the knee. The patients will receive ultrasound therapy for a minimum of 4 hrs each
day during normal activity (no doctors visits required). If successful, this
will potentially enable a pharmaceutical free approach to everyday pain relief.
Improved
Fetal Heart Rate Monitoring for Mothers and Doctors (Clinical Studies): Doppler
ultrasound has been used for over 20 years in measuring the fetal heart rate
(FHR) during labor and delivery of neonates. However, aside from switching the
signal processing from continuous-wave Doppler to pulse-wave Doppler FHR
monitoring in the late 1980s, few ultrasound advances have improved the field.
Our collaborator from Cayuga Medical Center, Dr. Steven Gelber,
found this frustrating and with our Cornell and Transducer Engineering Inc. research
team decided to improve FHR monitoring as well as uterine contraction
monitoring -- using ultrasound.
During
labor there is movement from the fetus inside the womb as well as from the
mother. Due to a very limited detection range, traditional ultrasound
transducers, which are strapped to the mothers belly, lose the heart rate
signal. The transducer must then be repositioned by the nurse to redetect the
FHR. Wireless telemetry devices for FHR monitoring exist, but FHR detection
only works well if the mother and fetus do not move. We have designed a custom
transducer that improves fetal tracking and spatial heart rate detection yet
works with existing FHR commercially available devices from GE and Philips Healthcare
(Figure 5).

Our
team has designed and tested the FHR monitoring transducer in the lab and is
now initiating patient testing at Cayuga Medical Center. Additionally, we have
begun development of an ultrasound based solution to measure the uterine
contraction strength and duration with a signal processing approach using the
same ultrasound transducer used for FHR monitoring. The overall goal is to
provide the doctor an ultrasound device to perform all heart rate and
contraction measurements throughout the labor and delivery and not require
repositioning.
The
future of ultrasound is truly amazing with unbounded possibilities, and it will
possibly cover the largest spectrum of medical related applications of any
other non-ionizing energy source. Our collaborative team of engineers and
clinicians seek to extend and improve ultrasound, and deliver the technology in
an easy to use, pocket-sized platform.
This
research is supported in part by the National Institutes of Health, the
National Science Foundation, Weill-Cornell Brain Tumor Project, EBL Products
Inc. and Transducer Engineering Inc.