The Biology
of Noise-Induced Hearing Loss Cochlear Nerve Loss After Reversible Acoustic
Injury
M. Charles Liberman - charles_liberman@meei.harvard.edu
Sharon G. Kujawa - sharon_kujawa@meei.harvard.edu
Eaton-Peabody
Laboratories and Departments of Otololaryangoloy and
Audiology
Massachusetts Eye
and Ear Infirmary, Boston MA 02114
Popular version
of paper 1aPP2
Presented Monday morning, April 19, 2010
159th ASA meeting, Baltimore MD
Overexposure to
loud sound can cause hearing loss: the severity depends on the level, duration
and frequency content of the exposure, as well as the vulnerability of the
listener. Noise-induced hearing loss (NIHL) is typically quantified by the
threshold audiogram, which measures the intensity to which tones of different
frequencies must be raised to be detectable. After an overexposure, thresholds
can be immediately elevated, but can recover for several weeks. If the
audiogram returns to normal, the NIHL is deemed reversible; if recovery is
incomplete after a few weeks, the NIHL is deemed permanent.
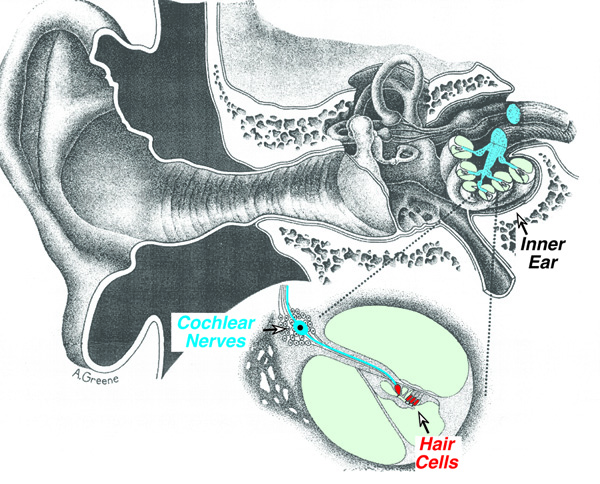
Most NIHL is
caused by damage to the inner ear (cochlea), specifically to the sensory cells
(hair cells), which convert sound-induced mechanical vibrations in the cochlear
fluids into electrical signals that are carried to the brain by fibers of the
cochlear nerve (Fig. 1). Temporary NIHL is caused by sub-microscopic changes to
the hair cells machinery that reversibly compromise its function. Permanent
NIHL is caused by hair cell death, which can occur within hours of exposure:
once hair cells die, they are never replaced (unless youre a bird, but thats
another story!). Degeneration of the cochlear nerve is much slower, with neural
loss continuing for months to years post exposure. This time delay has
suggested that hair cell damage is the primary effect of noise, and that nerve
degeneration occurs only when the hair cells are destroyed first.
Recent work in our
laboratory has shown that significant degeneration of the cochlear nerve occurs
after noise exposure, even when there is no hair cell loss, and even when
thresholds have returned to normal. We
study acoustic injury in mice and guinea pigs. Since the inner ears of all
mammals are very similar, findings in these rodents almost certainly apply to
humans. We expose animals to continuous noise for 2 hours at levels from 100 -
105 dB SPL, well below the threshold of pain, and roughly equivalent to the
noise produced by a belt sander or circular saw.
Before and after
exposure, we measure thresholds by two non-invasive techniques (also used in
the clinics to measure hearing in infants): 1) auditory brainstem responses
(ABRs), which are electrical potentials measured from scalp electrodes that
represent the summed activity of cochlear nerve fibers evoked by short tone
bursts; and 2) distortion product otoacoustic
emissions (DPOAEs), which are sounds created and amplified by normal hair cells
in response to a two-tone input sound that are propagated back out to the ear
canal where they can be measured with a sensitive microphone. Immediately after the noise exposure, our
animals show a moderate NIHL of 30 40 dB, by both ABRs and DPOAEs. Two weeks
later, thresholds have returned to normal, however, ABR amplitudes recover only
to < 50% of pre-exposure values, suggesting degeneration of >50% of the cochlear nerve.
At several
post-exposure times, we look at the microscopic structure of the inner ear.
Using antibodies to stain specific cellular components, we see that > 50% of
the synaptic connections between hair cells and neurons disappear within 1 day
after exposure. Although functionally disconnected from the hair cell, these
nerve fibers survive for many months. However, by two years post-exposure, the
nerve loss (~50%) matches the degree of ABR amplitude reduction, even though
all the hair cells remain intact. The slow neurodegeneration
is caused by disruption of the normal trophic support
these neurons receive from their cellular neighbors in the hair cell area.
Clearly, our work
challenges the long-held view that reversibility of noise-induced threshold
shifts indicates complete recovery of cochlear structures. It also suggests
that current damage risk criteria for human noise exposure may be inadequate,
because they are based on the assumption that reversible threshold shifts are
benign.
Is it paradoxical
that thresholds return to normal despite loss of > 50% of the nerve fibers
connecting hair cells to the brain? No - research from the 1950s in
behaviorally trained animals showed that partial lesions of the cochlear nerve
do not affect thresholds for detection of tones in quiet, as long as the hair
cells are functioning normally. What, then, are the functional consequences of
this primary neural degeneration? We believe that the neuronal loss will affect
hearing in a noisy environment and may explain why difficulties with hearing in
noise increase so dramatically in the aging ear.