Listening for the Pop in Ultrasound-mediated Drug
Delivery
Kirthi Radhakrishnan- radhakki@mail.uc.edu
Jonathan A. Kopecheck - kopechja@mail.uc.edu
Kevin J. Haworth - kevin.haworth@uc.edu
University of Cincinnati
231 Albert Sabin Way, MSB 6155
Cincinnati, OH, 45267
Shaoling Huang - Shaoling.Huang@uth.tmc.edu
David D. McPherson - David.D.McPherson@uth.tmc.edu
University of Texas Health Science Center at Houston
6431
Fannin, MSB 1.252,
Houston,
TX 77030
Christy K. Holland - christy.holland@uc.edu
University of Cincinnati
231 Albert Sabin Way, MSB 6155
Cincinnati, OH, 45267
Popular version of paper 4pBB5
Presented Thursday afternoon, April 22, 2010
159th ASA Meeting, Baltimore , MD
Ultrasound-mediated
drug delivery creates the potential for a new kind of treatment strategy, one
in which drugs are monitored as they travel through the patients body and are
released upon arrival at the target site.
This
is useful for drugs that might have toxic effects if released throughout the
body, or drugs targeted to a unique site in the vasculature, for example, a
clot-busting agent. By encapsulating these drugs into fluid- and gas-filled
liposomes, doctors can inject them into the bloodstream, monitor their presence
and then release them at a specific location with targeted ultrasound.
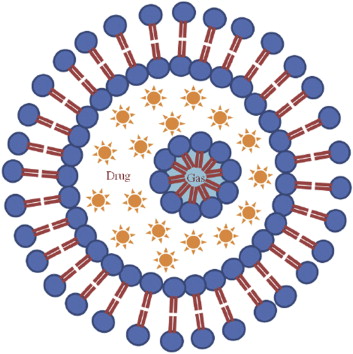
Figure 1. Schematic of an echogenic
liposome
The
novelty of this strategy lies in providing doctors with the capability to
control the amount of drug released and delivered at the target site, all
tailored to the patients needs. This can be achieved by manipulating the way
ultrasound pulses interact with the liposomes. The nature of this interaction
is governed by the gas within the liposomes, which makes them echogenic,
meaning that they are highlighted on an ultrasound image.
In
previous publications, we have shown that a clot-busting drug can be
encapsulated in echogenic liposomes
and released using diagnostic Doppler ultrasound provided by a clinical
scanner. This release was found to be associated with the disappearance of
contrast on ultrasound images.
In
this work, the sensitivity of the echogenic liposomes to a variety of types of
ultrasound was studied. Depending on the amplitude of the Doppler ultrasound
pulses, the liposomes can respond in three possible ways: the intact vesicles
may be pushed away, the vesicles may gently ring (stable cavitation), or
the vesicles can be violently popped (inertial cavitation).
Both
of the cavitation reactions have been found to enhance delivery of drugs and
genes across cell walls and tissues. Stable cavitation seems to massage the
cells, coaxing them to open up temporarily and thus allow drug delivery without
destroying the cells. Inertial cavitation, on the other hand, seems to have the
ability to poke a more permanent hole in the cell, which can potentially kill
the cell.
In
order to achieve the desired therapeutic effect and to examine the possibility
of negative bioeffects, we needed to find the sweet spot, or pressure
amplitude at which the ultrasound would gently shake the bubbles but not
violently pop them. Fortunately, each
type of cavitation results in a characteristic echo. Thus by recording the echoes, in addition to
simultaneously capturing standard ultrasound images, we can begin to understand
how the imaging of the echogenic liposomes may be coupled to their biological
effect.
The
experiments were performed by infusing the liposomes into a flow system
consisting of a pump and tubing that mimic blood flow in an artery. The
liposomes were hit with ultrasound pulses, similar to those that doctors use to
listen to the heartbeat of a fetus. A
separate ultrasound listening device recorded the echoes. Simultaneously, we
recorded the loss of contrast in an ultrasound image that resulted from the
ultrasound interacting with the liposomes.
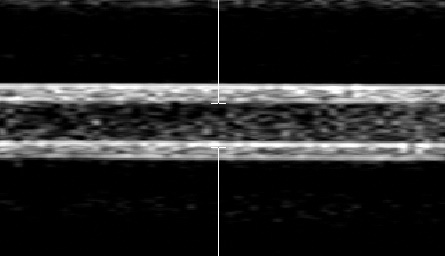
Figure 2. Image of echogenic liposomes flowing in a
latex tube hit by ultrasound Doppler pulses at the center (indicated by white
vertical bars). Flow is from right to left. Disappearance of contrast of echogenic liposomes on the
left-side of the image, after being hit by Doppler ultrasound, can be seen.
As
the amplitude of the ultrasound pulses is stepped up gradually, the ringing
effect was found to increaseindicating that stable cavitation sets in at low
amplitude and continues to occur even at higher amplitudes. On the other hand,
the ultrasound pulses begin to pop the vesicles substantially only at higher
amplitudes, indicating that there is a sweet spot where stable cavitation
occurs without inertial cavitation setting in. Concurrent with the popping of
the vesicles, there is also a higher disappearance of contrast on the
ultrasound images.
In
further studies, we will examine how the stable cavitation and inertial
cavitation activities correlate with the amount of drug release from echogenic
liposomes and the delivery of these drug molecules into blood vessels. We will
also examine the vessels for cellular damage and optimize the range of
amplitudes over which the drug release and delivery is enhanced without
negative biological effects.