A New Acoustic
Lens Design for Electromagnetic Shock Wave Lithotripters
Nathan Smith - nathan.b.smith@duke.edu
Dept.
of Mechanical Engineering and Materials Science, Duke Univ.
144
Hudson Hall, Box 90300, Durham, NC 27708
W.
Neal Simmons, Georgy N. Sankin,
Pei Zhong
Dept.
of Mechanical Engineering and Materials Science, Duke Univ.
Durham,
NC 27708
Popular
version of paper 1pBB11
Presented
Monday afternoon, April 19, 2010
159th
ASA Meeting, Baltimore, MD.
Extracorporeal
shock wave lithotripsy (ESWL) is a clinically preferred technique for treatment
of kidney stone disease involving the use of focused acoustic shock waves to
obliterate stones inside a patient. A
key advantage ESWL has over other stone treatment methods is its noninvasiveness,
i.e. surgery is unnecessary. Many
different designs exist to convert low-energy density acoustic pulses (over a
large surface area) outside a patient into high-energy density focused shock
waves (over a small area) inside a patient; one clinically relevant model known
as the electromagnetic (EM) lithotripter uses, in essence, a loudspeaker fitted
with a lens for acoustic focusing. Other
clinical lithotripters employ electrohydraulic (EH) and
piezoelectric technologies.
The
1st-generation lithotripter, known as the Dornier HM-3, utilizes EH
technology for shock wave generation and a truncated ellipsoidal reflector for
wave focusing. The HM-3 machine is
characterized by its low peak pressure and wide focal width. Development of the subsequent 2nd-
and 3rd-generation machines erroneously centered on narrowing the
lithotripter focal width and increasing peak pressure to maximize stone targeting
and destructive capacity. Unfortunately,
patient respiration and stone spreading in the kidney collecting system can produce
elusive and widespread stone targets.
Furthermore, conventional fluoroscopic (x-ray) imaging does not allow
for visualization and targeting of smaller stones. Currently, ESWL researchers and urologists are
again noticing the benefit in stone fragmentation efficiency using wide focal
width lithotripters.
Another
desirable characteristic of the Dornier HM-3, as well as other EH lithotripters,
is its unique wave shape. Figure 1
illustrates how EH lithotripters produce strong compressive shock waves and
long trailing tensile regions, whereas EM and piezoelectric lithotripters
produce shock wave profiles with ringing of compression and tension phases, similar
to a damped oscillator.
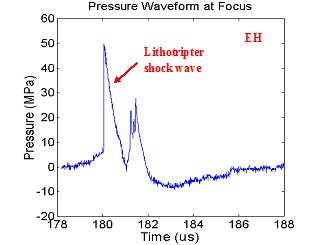
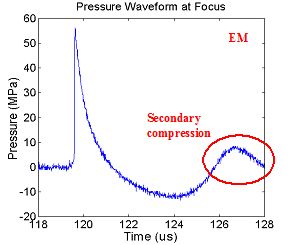
Figure 1. (A)
Representative electrohydraulic (EH) lithotripter
waveform. (B) Representative
electromagnetic (EM) lithotripter waveform demonstrating secondary compression
from ringing (circled).
The
impact of ringing is on bubble activity, a known mechanism of stone
fragmentation. Tension from the trailing
region of the lithotripter pulse can induce sub-micron-sized gas pockets in the
urine to grow into millimeter-sized bubbles.
After the passage of the lithotripter pulse these bubble collapse, often
generating highly localized pressure on the order of the lithotripter shock
wave. Compression ringing in the urine
can act to lessen the growth of bubbles, thereby lessening their collapse impact
on the surface of stones.
A
novel acoustic lens design applicable to EM lithotripters has been developed as
a means to simultaneously increase bubble activity in the focal region through
reduction of secondary compression while broadening the lithotripter focal
width. Through a ring cut along the periphery
of the acoustic lens, the effective aperture of the lithotripter is reduced,
which inversely affects its focal size. The
lens cut also acts to delay a portion of the acoustic pulse as it approaches
the focusing lens, as can be seen in Figure 2.
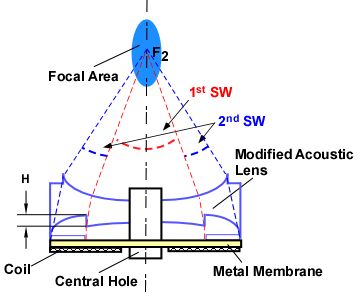
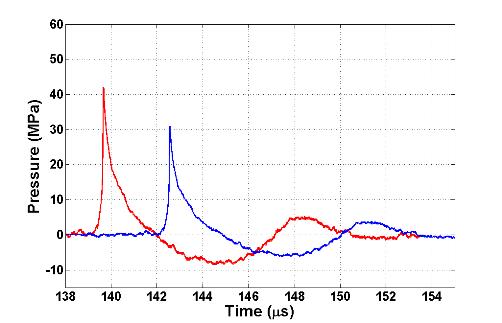
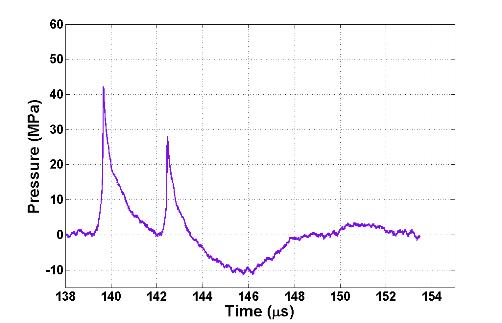
Figure 2. (A) EM lithotripter
cross-section demonstrating pulse superposition. (B) Theoretical representation of pulse
superposition as component waveforms.
(C) The theoretical combined superposed waveform.
This
delayed pulse superposes on the unmodified shock wave in such a way that it
creates destructive interference and flattens the ringing present in its shock
wave profile. Figure 3 shows actual
waveforms from this destructive interference compared to the original lens
design as well as an energy density plot to illustrate the beam widening
effect.
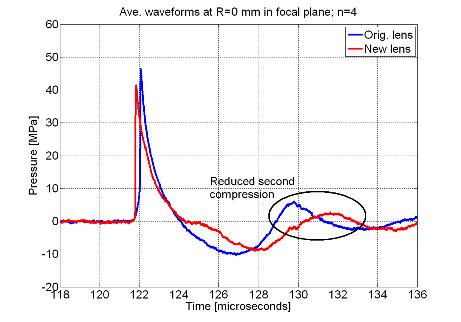
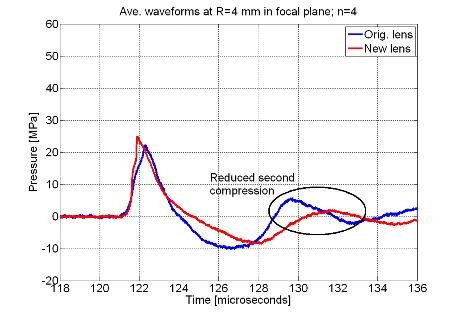
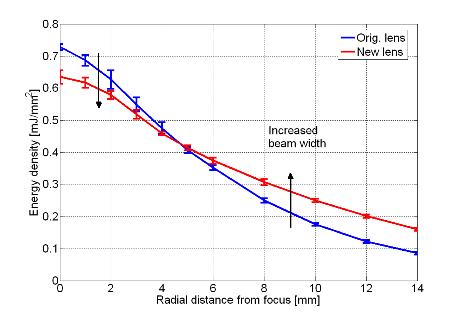
Figure 3. (A) Comparison
between original and new lens waveforms at the focus and (B) 4 mm away in the
focal plane to demonstrate actual reduction of second compression. (C) Energy density comparison showing widened
beam with new lens.
In vitro phantom tests and
in vivo animal studies in a swine
model have shown promising results with the new lens design. Experiments were conducted with both the
original and new lenses at equivalent focal acoustic energies in a radius encompassing
most clinical kidney stones treated with ESWL as well as the phantom kidney
stones used for this study. With a nearly
50% wider beam diameter than the original source, the EM lithotripter with new
lens produced statistically higher stone fragmentation efficiency for
clinically relevant doses in the swine model as well as for idealized in vitro conditions detailed in Figure 4.
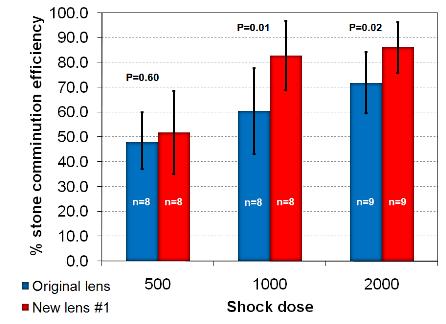
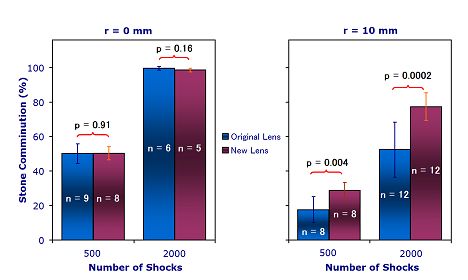
Figure 4. (A)
Fragmentation efficiency in a swine model for 500, 1000 and 2000 shock
doses. (B) Fragmentation efficiency in a
flat-base tube holder at 2 different positions in the focal plane (focus and 10
mm from focus) and 2 different shock doses (500 and 2000).]
Lens
optimization and fragmentation efficiency assessment phases have concluded, and
the new lens for EM lithotripters is presently undergoing evaluation for tissue
injury potential in comparison with the original lens. Before long, the new lens will be implemented
and evaluated clinically.