Matthew O’Donnell – odonnell@uw.edu (Can be reached by cell at 206-465-2144)
Xiaohu Gao – xgao@u.washington.edu
Department of Bioengineering
William H. Foege Building
Box 355061
3720 15th Ave NE
University of Washington
Seattle, WA 98195-5061
Popular version of paper 5aBA5
Presented at the 161st ASA Meeting, Seattle, Washington
Cancer metastasis is generally caused by cancer cells moving through the blood stream and subsequent growth of tumor cells at distant organs. Circulating tumor cells (CTCs) have been recommended by the American Society of Clinical Oncology as important tumor biomarkers. Real-time monitoring of CTCs is equally urgent to evaluate the therapeutic effectiveness of individually tailored treatments. At present, the success or failure of anticancer therapies is only assessed retrospectively by the absence or presence of metastasis. However, metastatic disease is incurable by any current therapy. Therefore, real-time monitoring of CTCs during and after therapy could provide unique information for clinical management of individual cancer patients, and allow an early change in therapy years before the appearance of incurable metastasis. Detection and isolation of CTCs combined with molecular characterization are of tremendous value for cancer patients.
Despite this urgent need, no robust technologies exist for detecting and treating low concentrations of CTCs. In particular, imaging technologies are not effective for detection of these low-abundance cells because of the poor image contrast even with highly specific, targeted molecular contrast agents. In recent work for our labs, we have invented a new molecular imaging modality, magnetomotive photoacoustic imaging (mmPA), with significantly improved specific molecular contrast (Y. Jin, C. Jia, S-W Huang, M. O’Donnell, and X. Gao, Nature Communications 1:41, 8 pages, July, 2010). By synchronizing photoacoustic data acquisition with magnetic field-triggered motion (Fig. 1), advanced signal processing methods can improve specific molecular contrast by at least 2-3 orders of magnitude, providing a unique opportunity to detect and quantify low-abundance CTCs in blood circulation. This technology integrates an intelligent combination of PA contrast agents (NIR light absorbing Au nanoshell) with magnetic NPs (Fe3O4, responsive to external oscillating magnetic fields) into a single nanoparticle. In addition, the magnetic component of these particles enables magnetic trapping of CTCs to isolate even low concentrations of these cells for targeted hyperthermal therapy in vivo. For clinical translation of this powerful technology, we will explore an integrated approach combining biocompatible contrast agents, mmPA imaging, magnetic trapping, and targeted thermal therapy technologies with a modern real-time ultrasound system for robust detection, isolation, and treatment of CTCs.
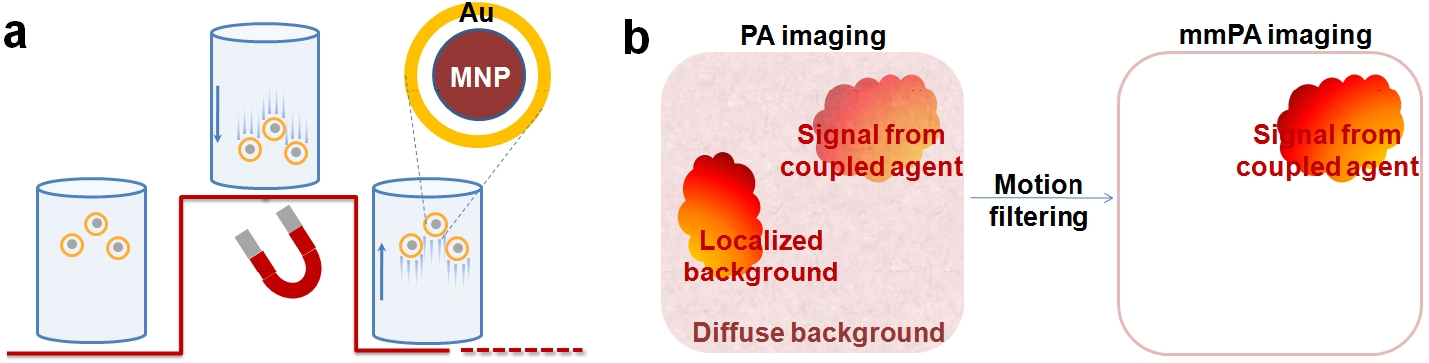
Fig. 1. Schematic of mmPA. (a) MNP-Au core-shell NPs’ response to a magnetic field. The underlying curve represents field strength. The coupled agents move as the magnetic field is turned on and off. (b) Contrast enhancement. mmPA imaging suppresses regions not susceptible to a controlled magnetic field while identifying regions with coupled agents responsive to a magnetic field. Nat. Commun. 2010