Biomedical ultrasound: Making a Quantum Leap in Medicine
Tyrone Porter, Ph.D.
Boston University
Department of Mechanical Engineering
Department of Biomedical Engineering
Center for Nanoscience and Nanobiotechnology
Combining creativity and ingenuity, scientists are expanding the role of ultrasound in the clinical setting. Historically, ultrasound has been used for such applications as imaging fetal development or quantifying blood flow. In recent developments, researchers are fusing expertise in physics, biochemistry, cell and molecular biology, and nanotechnology to formulate solutions to tough clinical problems. The collaborative spirit has led to the production of specially engineered particles for novel imaging and therapeutic applications. These particles include monodisperse populations of coated microbubbles and pressure- and temperature-sensitive nanoparticles for triggered delivery of therapeutic agents.
Targeted imaging with monodispersions of coated microbubbles

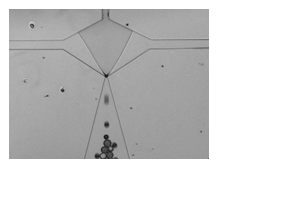
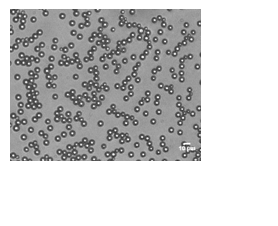 Coated microbubbles have been used clinically as ultrasound contrast agents for more than two decades. By incorporating targeting moieties, such as antibodies or peptides, in the outer shell, the microbubbles can be targeted to receptors overexpressed on the membranes of diseased cells. This approach, known as molecular imaging, provides vital information about the structure and underlying processes at the molecular level that contribute to the evolution of a disease. The key to this technique is the detection of the bound microbubbles with diagnostic ultrasound. It has been predicted theoretically that narrowly distributed coated microbubbles (i.e. monodisperse) are more echogenic than broadly distributed microbubbles when excited close to resonance. Based upon these theoretical predictions, researchers have begun to novel procedures to produce monodisperse populations of ultrasound contrast agents (Hettiarachchi et al. 2006; Farook et al. 2007; Hettiarachchi et al. 2007). For example, my group is manufacturing microfluidic devices to channel gas and lipid solutions through an orifice, producing lipid-coated microbubbles with a very narrow size distribution (Figure 1). Alternative approaches to producing monodispersions include differential centrifugation (Feshitan et al. 2009) and electrohydrodynamic atomization (Farook et al. 2009). Recent experiments performed by Streeter et al demonstrated that monodisperse microbubbles were detected with greater sensitivity by diagnostic ultrasound than polydisperse microbubbles, confirming the theoretical predictions (Streeter et al. 2010). Additional studies are underway to investigate the relationship between the size and composition of the outer shell with the resonance frequency for monodispersions of coated microbubbles. The knowledge gained will help scientists to tailor ultrasound contrast agents for a specific imaging frequency, thus improving the performance and utility of contrast-enhanced diagnostic ultrasound.
Coated microbubbles have been used clinically as ultrasound contrast agents for more than two decades. By incorporating targeting moieties, such as antibodies or peptides, in the outer shell, the microbubbles can be targeted to receptors overexpressed on the membranes of diseased cells. This approach, known as molecular imaging, provides vital information about the structure and underlying processes at the molecular level that contribute to the evolution of a disease. The key to this technique is the detection of the bound microbubbles with diagnostic ultrasound. It has been predicted theoretically that narrowly distributed coated microbubbles (i.e. monodisperse) are more echogenic than broadly distributed microbubbles when excited close to resonance. Based upon these theoretical predictions, researchers have begun to novel procedures to produce monodisperse populations of ultrasound contrast agents (Hettiarachchi et al. 2006; Farook et al. 2007; Hettiarachchi et al. 2007). For example, my group is manufacturing microfluidic devices to channel gas and lipid solutions through an orifice, producing lipid-coated microbubbles with a very narrow size distribution (Figure 1). Alternative approaches to producing monodispersions include differential centrifugation (Feshitan et al. 2009) and electrohydrodynamic atomization (Farook et al. 2009). Recent experiments performed by Streeter et al demonstrated that monodisperse microbubbles were detected with greater sensitivity by diagnostic ultrasound than polydisperse microbubbles, confirming the theoretical predictions (Streeter et al. 2010). Additional studies are underway to investigate the relationship between the size and composition of the outer shell with the resonance frequency for monodispersions of coated microbubbles. The knowledge gained will help scientists to tailor ultrasound contrast agents for a specific imaging frequency, thus improving the performance and utility of contrast-enhanced diagnostic ultrasound.
Therapeutic applications with high-intensity focused ultrasound
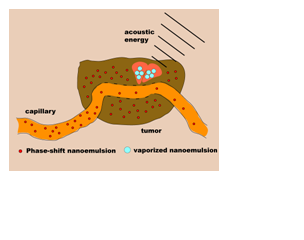

Using focused ultrasound transducers, acoustic energy can be concentrated at deep-tissue sites, such as the liver, with minimal losses along the propagation path. As a result, large pressure and temperature changes can be achieved without the insertion of probes into the tissue (i.e. noninvasive). There is considerable evidence that focused ultrasound can be used to accelerate the clotting process for sealing punctures and tears in deep-seated blood vessels that are difficult to close without surgery (Vaezy et al. 1998; Martin et al. 1999; Noble et al. 2002; Vaezy et al. 2004). More recently, researchers have used focused ultrasound as the cornerstone for a variety of novel approaches to treating cancer. Scientists at the University of Michigan have pioneered a method known as histotripsy to ablate tissue (Xu et al. 2007; Xu et al. 2007). In this approach, high-amplitude pressure waves transmitted by focused ultrasound are used to grind up and liquefy cancerous tissue without damaging the surrounding or intervening healthy tissue. Scientists at the University of Utah are developing pressure-sensitive submicron droplets for localized delivery of chemotherapy (Rapoport et al. 2009). The droplets are loaded with a cytotoxic drug, which is released upon vaporization with high-amplitude acoustic pulses (Rapoport et al. 2010). Finally, ultrasound can heat tissue due to absorption of acoustic energy. Tissue heated above 60oC results in crosslinking of intracellular proteins and ultimately cell death. This process, known as thermal ablation, can be accelerated with microbubbles. My group has developed submicron liquid droplets than can be vaporized locally with high-amplitude acoustic pulses, forming bubbles that are used to accelerate ultrasound absorption and thermal ablation (see Figure 2). Alternatively, ultrasound can be used to heat tissue to a lower temperature (~ 43oC) and trigger release of drugs entrapped within temperature-sensitive liposomes (see animation). This approach has been implemented successfully for localized delivery of chemotherapy within solid tumors (Dromi et al. 2007). Due to its versatility, ultrasound provides clinicians with many options for diagnosing and treating diseases. At the dawn of a new era in medicine, the future of biomedical acoustics is bright.
Animation
Animation Caption. Focused ultrasound waves heats tissue containing drug-loaded temperature-sensitive liposomes. When the temperature exceeds a predefined threshold (i.e. 43oC), the lipid shell melts and the entrapped drug (red drops) is released."
Dromi, S., V. Frenkel, et al. (2007). "Pulsed-high intensity focused ultrasound and low temperature sensitive liposomes for enhanced targeted drug delivery and antitumor effect." Clinical Cancer Research 13(9): 2722-2727.
Farook, U., E. Stride, et al. (2009). "Controlling size and size distribution of electrohydrodynamically prepared microbubbles." Bubble Science, Engineering and Technology 1: 53-55.
Farook, U., H. B. Zhang, et al. (2007). "Preparation of microbubble suspensions by co-axial electrohydrodynamic atomization." Medical Engineering & Physics 29(7): 749-754.
Feshitan, J. A., C. C. Chen, et al. (2009). "Microbubble size isolation by differential centrifugation." Journal of Colloid and Interface Science 329(2): 316-324.
Hettiarachchi, K., P. Dayton, et al. (2006). "Formulation of Monodisperse Contrast Agents in Microfluidics Systems for Ultrasound Imaging." Proceedings of 2006 International Conference of Microtechnologies in medicine and Biology: 230-232.
Hettiarachchi, K., E. Talu, et al. (2007). "On-chip generation of microbubbles as a practical technology for manufacturing contrast agents for ultrasonic imaging." Lab on a Chip 7(4): 463-468.
Martin, R. W., S. Vaezy, et al. (1999). "Hemostasis of punctured vessels using Doppler-guided high-intensity ultrasound." Ultrasound Med Biol 25(6): 985-990.
Noble, M. L., S. Vaezy, et al. (2002). "Spleen hemostasis using high-intensity ultrasound: survival and healing." J Trauma 53(6): 1115-1120.
Rapoport, N., D. A. Christensen, et al. (2010). "Cavitation properties of block copolymer stabilized phase-shift nanoemulsions used as drug carriers." Ultrasound Med Biol 36(3): 419-429.
Rapoport, N. Y., A. M. Kennedy, et al. (2009). "Controlled and targeted tumor chemotherapy by ultrasound-activated nanoemulsions/microbubbles." J Control Release 138(3): 268-276.
Streeter, J. E., R. Gessner, et al. (2010). "Improving sensitivity in ultrasound molecular imaging by tailoring contrast agent size distribution: in vivo studies." Mol Imaging 9(2): 87-95.
Vaezy, S., R. Martin, et al. (1998). "Hemostasis of punctured blood vessels using high-intensity focused ultrasound." Ultrasound Med Biol 24(6): 903-910.
Vaezy, S., M. L. Noble, et al. (2004). "Liver hemostasis with high-intensity ultrasound: repair and healing." J Ultrasound Med 23(2): 217-225.
Xu, Z., T. L. Hall, et al. (2007). "Effects of acoustic parameters on bubble cloud dynamics in ultrasound tissue erosion (histotripsy)." J Acoust Soc Am 122(1): 229-236.
Xu, Z., M. Raghavan, et al. (2007). "High speed imaging of bubble clouds generated in pulsed ultrasound cavitational therapy--histotripsy." IEEE Trans Ultrason Ferroelectr Freq Control 54(10): 2091-2101.


 Coated microbubbles have been used clinically as ultrasound contrast agents for more than two decades. By incorporating targeting moieties, such as antibodies or peptides, in the outer shell, the microbubbles can be targeted to receptors overexpressed on the membranes of diseased cells. This approach, known as molecular imaging, provides vital information about the structure and underlying processes at the molecular level that contribute to the evolution of a disease. The key to this technique is the detection of the bound microbubbles with diagnostic ultrasound. It has been predicted theoretically that narrowly distributed coated microbubbles (i.e. monodisperse) are more echogenic than broadly distributed microbubbles when excited close to resonance. Based upon these theoretical predictions, researchers have begun to novel procedures to produce monodisperse populations of ultrasound contrast agents (Hettiarachchi et al. 2006; Farook et al. 2007; Hettiarachchi et al. 2007). For example, my group is manufacturing microfluidic devices to channel gas and lipid solutions through an orifice, producing lipid-coated microbubbles with a very narrow size distribution (Figure 1). Alternative approaches to producing monodispersions include differential centrifugation (Feshitan et al. 2009) and electrohydrodynamic atomization (Farook et al. 2009). Recent experiments performed by Streeter et al demonstrated that monodisperse microbubbles were detected with greater sensitivity by diagnostic ultrasound than polydisperse microbubbles, confirming the theoretical predictions (Streeter et al. 2010). Additional studies are underway to investigate the relationship between the size and composition of the outer shell with the resonance frequency for monodispersions of coated microbubbles. The knowledge gained will help scientists to tailor ultrasound contrast agents for a specific imaging frequency, thus improving the performance and utility of contrast-enhanced diagnostic ultrasound.
Coated microbubbles have been used clinically as ultrasound contrast agents for more than two decades. By incorporating targeting moieties, such as antibodies or peptides, in the outer shell, the microbubbles can be targeted to receptors overexpressed on the membranes of diseased cells. This approach, known as molecular imaging, provides vital information about the structure and underlying processes at the molecular level that contribute to the evolution of a disease. The key to this technique is the detection of the bound microbubbles with diagnostic ultrasound. It has been predicted theoretically that narrowly distributed coated microbubbles (i.e. monodisperse) are more echogenic than broadly distributed microbubbles when excited close to resonance. Based upon these theoretical predictions, researchers have begun to novel procedures to produce monodisperse populations of ultrasound contrast agents (Hettiarachchi et al. 2006; Farook et al. 2007; Hettiarachchi et al. 2007). For example, my group is manufacturing microfluidic devices to channel gas and lipid solutions through an orifice, producing lipid-coated microbubbles with a very narrow size distribution (Figure 1). Alternative approaches to producing monodispersions include differential centrifugation (Feshitan et al. 2009) and electrohydrodynamic atomization (Farook et al. 2009). Recent experiments performed by Streeter et al demonstrated that monodisperse microbubbles were detected with greater sensitivity by diagnostic ultrasound than polydisperse microbubbles, confirming the theoretical predictions (Streeter et al. 2010). Additional studies are underway to investigate the relationship between the size and composition of the outer shell with the resonance frequency for monodispersions of coated microbubbles. The knowledge gained will help scientists to tailor ultrasound contrast agents for a specific imaging frequency, thus improving the performance and utility of contrast-enhanced diagnostic ultrasound.
