Stuart Ibsen - sibsen@ucsd.edu
Michael Benchimol
Dmitri Simberg
Carolyn Schutt
Jason Steiner
Sadik Esener
University of California San Diego
3855 Health Sciences Dr. # 0815
La Jolla, CA 92093-0815
Popular version of paper 4aBA12 “Localized Activation and Cellular Effects of Ultrasound Triggered Drug Delivery Vehicles with Encapsulated Microbubbles”
Presented Thursday morning, November 3, 2011
162nd ASA Meeting, San Diego, Calif.
The body contains a variety of cell types which need to replicate quickly as part of their normal healthy function. Some examples include hair follicles, the lining of your stomach and even cells involved with the immune system. In each of these cases the replication is tightly controlled to specified locations. In contrast, tumor cells are so dangerous because they divide rapidly, but do so in an unregulated and uncontrolled manner and can spread everywhere. These properties of tumor cells have been known for over 70 years and most chemotherapy agents are designed to kill cells that replicate. The trouble is that these drugs by themselves cannot distinguish between tumor cells and healthy cells. Any cell in the body that tries to replicate will have trouble and could be damaged or killed by the treatment. This leads to the side effects that people must endure while on chemotherapy including the short-term effects of nausea and hair loss. Long term effects can occur years after treatment has ended and include conditions such as heart problems and even the occurrence of new tumors. Another complication is that the chemotherapy drugs weaken the immune system. The immune system is supposed to play a significant role in eliminating the tumor from the body and the chemotherapy interferes with this ability and can work against the patient. The next big leap in chemotherapy is to design a treatment that will allow the chemotherapy drugs to act only on the tumor cells leaving the healthy cells and immune system alone.
One method to avoid healthy cells coming into contact with the injected chemotherapy dose is to encapsulate the drug at high concentrations within a tiny vehicle. These vehicles can be injected by the millions into the bloodstream with very little effect on healthy tissue. The surface of the vehicle can be modified to allow them to circulate within the vasculature for a long time allowing a large number of vehicles to pass through the blood vessels of the tumor. However, these vehicles are unable to distinguish between the tumor and the healthy tissue and thus hold onto the drug even as they pass through the tumor, greatly reducing their therapeutic effect
To address this challenge Dr. Sadik Esener and Dr. Stuart Ibsen at the UC San Diego Moores Cancer Center have taken a multi-layer approach, creating vehicles that are structured much like the layers of an onion. Dr. Esener’s multidisciplinary lab has created vehicles where the outer layers convey long circulation properties and the inner layers have efficient tumor penetration. This means that the outer layer must be removed when the vehicle gets inside the tumor. The removal has to be done very fast, and must not occur in healthy tissue. Trying to use the biochemical environment of the tumor alone to remove the layer can be challenging, since the tumor environment often shows very little difference from healthy tissue. There is no reliable way to tweak the surface of the vehicle to chemically open up just in the tumor. For the vehicle to be triggered only within the tumor there must be a substantial and predictable difference between the tumor and the healthy tissue which can be artificially created by highlighting just the tumor region with focused ultrasound. Instead of using chemistry to remove the outer layer, the physical waves of the ultrasound can be used. The vehicles will only open up in the tumor, since the ultrasound intensity will be negligible everywhere else in the body.
To allow the ultrasound to physically remove layers while keeping within safe intensity levels one can take advantage of microbubbles of gas which are known to act as ultrasound antennas. These bubbles undergo size oscillations which can be used to open vehicles while keeping the ultrasound intensity below levels that would cause excessive tissue heating or damage. Esener’s lab has now demonstrated a new spin on the use of microbubbles by encapsulating them inside the vehicles themselves along with a chemotherapy drug

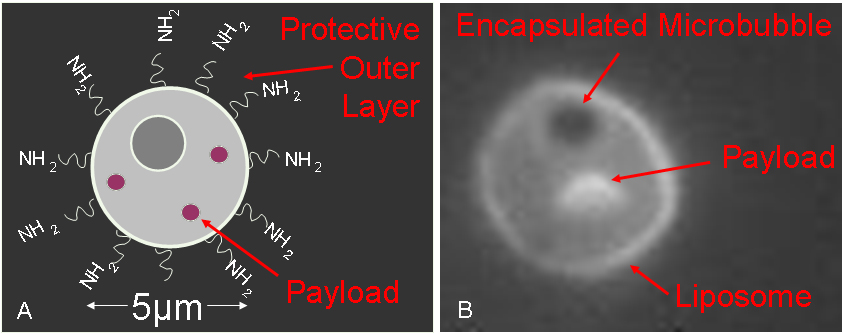
(“Figure 1. (A) Schematic representation of the drug delivery vehicle. (B) Actual fluorescent image of the vehicle with encapsulated microbubble.”).
This new method of microbubble encapsulation creates a stable structure where the microbubble freely bounces around inside
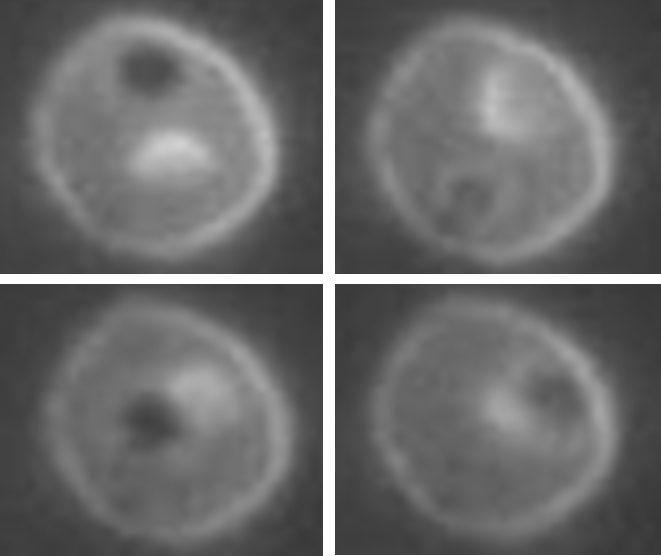
(“Figure 2. Sequence of four pictures showing the microbubble bouncing around the inside of the vehicle’s protective outer membrane.”).
Upon exposure to low intensity ultrasound, the microbubble creates a localized shockwave that radiates out and ruptures the vehicle’s outer membrane, triggering a pinpoint burst release of the contents with forceful jet-like patterns. The lab built a custom system which combines high-speed videography and fluorescent microscopy with focused ultrasound to record movies of these shockwave interactions with the delivery vehicle membranes and live cells, showing for the first time the actual burst release from the vehicle.
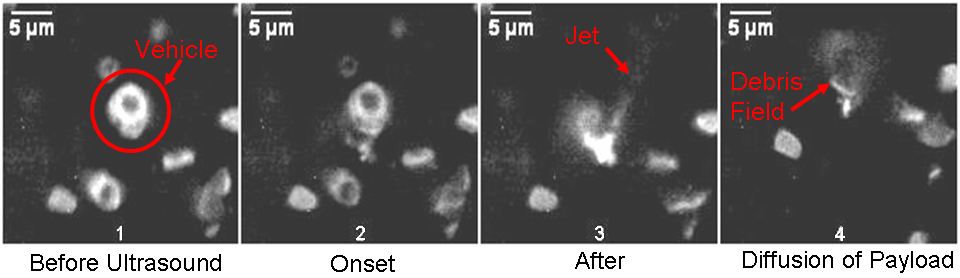
(“Figure 3. Still frame images taken from a movie of the shockwave explosion. The vehicle outer membrane is completely shattered.”)
If these vehicles can concentrate in the blood vessel of the tumor, focused ultrasound could create a huge burst release of chemotherapy that would have a very significant localized effect just inside the tumor itself. This was demonstrated in a simulated tumor sample.
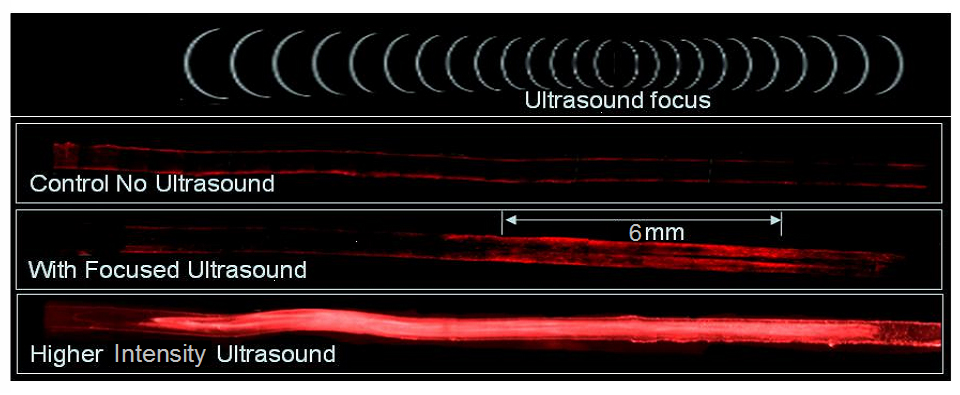
(“Figure 4. A simulated tissue sample with a blood vessel shows the activation of vehicles and delivery of contents in just the ultrasound focal region. This is what it might look like inside of a real tumor blood vessel.”) This externally-activated scheme could lead to truly tumor-specific drug delivery.
NCI Grant No. 5U54CA119335-05, and UCSD Cancer Center Specialized Support Grant P30 CA23100 supported this work.