Breast cancer impacts 12.2% of women in the United States, with a third of all patients dying from the disease. Approximately half of breast cancer patients choose a procedure known as lumpectomy for major part of their treatment to remove the malignant (cancerous) tissue. Surgeons strive to obtain negative margins (cancer-free tissues surrounding the tumor) in surgery to prevent further malignancy in the treated breast. Nevertheless, 30-50% of lumpectomy patients require additional surgery due to positive margins.
An interdisciplinary team of scientists and medical doctors from three Utah universities and the Huntsman Cancer Institute are developing advanced ultrasonic technology for surgical use that will assist surgeons in removing microscopic cancer from the breast during procedures such as lumpectomy. The new technique promises to reduce the high number of repeat surgeries after lumpectomy by detecting and identifying cancerous tissues along the edges, rapidly and in real-time. This will allow the surgeon to accurately remove enough tissue to ensure no cancer remains, while preserving as much unaffected tissue as possible.
The new method uses ultrasonic frequencies that are much higher than currently used in standard medical ultrasound to classify the pathology and identify breast tissue health. In addition to these higher frequencies, new state-of-the-art data analysis methods are being developed and tested. Sophisticated computer programs that simulate the interaction of ultrasound with breast tissue at the microscopic level are used to differentiate normal from cancerous cells. The synergy of these innovative techniques provides a powerful tool for surgeons to improve the care of breast cancer patients using a simple, portable, and cost-effective ultrasonic system.
A feasibility study funded by the National Cancer Institute of the National Institutes of Health was recently completed at the Huntsman Cancer Institute of the University of Utah. The study included 34 specimens from 17 patients, each of whom had been diagnosed with breast cancer. Each tissue specimen was ultrasonically tested immediately following removal in surgery and before pathology analysis. These tissue samples were classified into 15 pathology types. Each tissue was analyzed for specific ultrasonic signal correlations using medical statistical methods and computer simulation. The preliminary data shows promising correlations between a wide range of breast tissue pathologies. These include normal tissue, benign conditions (such as Fat-Adenomas and Fibrocystic changes), and malignant disease (such as Invasive Ductal Carcinoma(IDC)). Initial sensitivities and specificities are comparable to current methods in use or under development. However, the high-frequency ultrasonic method is faster and provides the surgeon information that can impact the immediate care of the patient in the operating room.
Figure 1. Portable ultrasonic system used in breast tissue analysis at the Huntsman Cancer Institute.
New ultrasonic parameters discovered in this study are sensitive to the microscopic structure of breast tissue glands. These parameters not only allow malignant pathologies to be distinguished from benign conditions, but may also permit different types of cancer to be identified based on their microscopic ultrasonic properties. As a result, this new ultrasonic technology has significant potential for differentiating between different types of cancer. Many other methods being researched are incapable of similar selectivity. Beyond the treatment of breast cancer patients, our technique can also be applied to Moh’s surgery (the technique used to assure complete removal of skin cancers) and any other cancer or disease where obtaining tumor-free margins is an important part of the treatment.
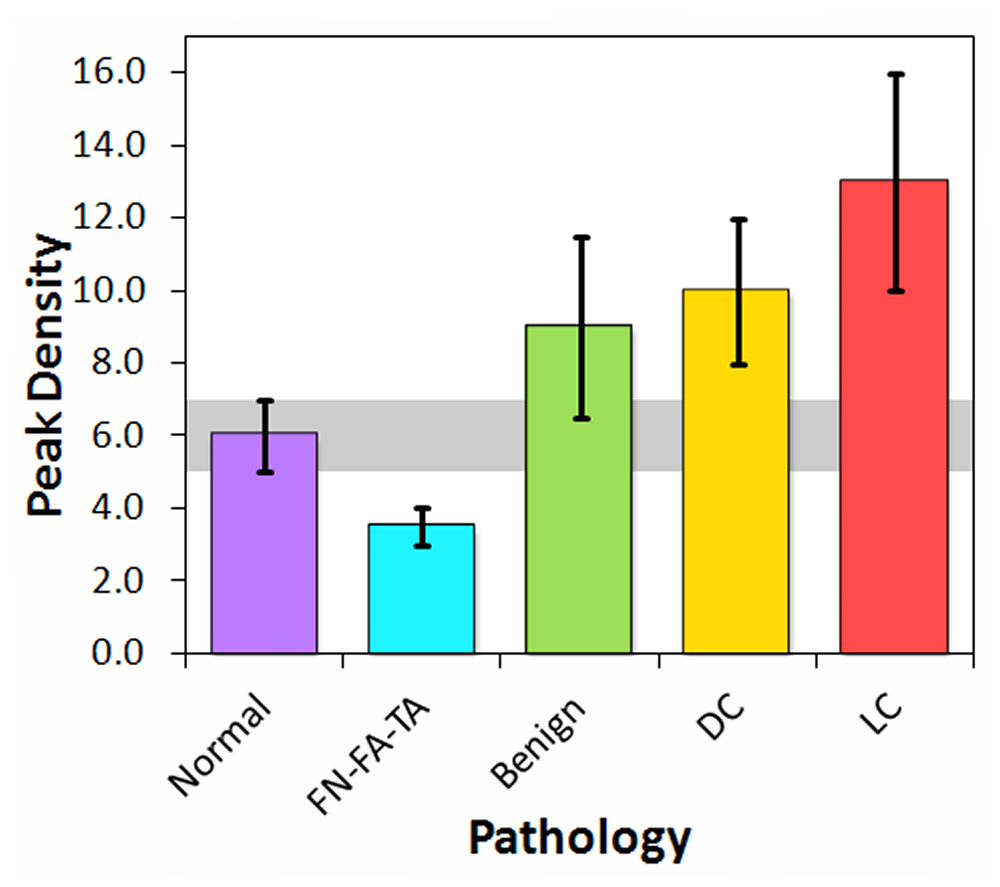
Figure 2. Peak densities measured at frequencies of 20 to 80 MHZ for tissue classifications employed in differentiating between cancer pathologies. [FN-FA-TA: fat necrosis–fibroadenoma–tubular adenoma; DC: ductal carcinomas (DCIS & IDC); LC: lobular carcinomas (LCIS & ILC)]
New data analysis methods supply greater detail that can be utilized in distinguishing between many breast pathologies, thus providing surgeons with new information to operate with greater accuracy. For example, preliminary results visibly display the potential of the ultrasonic method to ascertain the presence of Lobular Carcinoma (LC). As shown in Figure 2, the peak density of LC (an ultrasonic parameter derived from data analysis and computer simulation) is greater than that of other pathologies. The sensitivity of the high-frequency ultrasonic data to LC (particularly Invasive Lobular Carcinoma (ILC)) has immense surgical implications since LC is one of the most difficult cancers to fully remove in a lumpectomy.
Part of the continuing research includes improving the data analysis methods by looking at various combinations of data sets. One example includes comparing the calculated attenuation (decrease in amplitude) and peak density (concentration of “waves”) of an unknown specimen against a specific pathology. High-frequency array methods are also being explored to improve the imaging capabilities of this technique. Further testing of surgical specimens will likewise help to increase the accuracy and reliability of the method.
Figure 3. A three-dimensional rendition of simulated breast tissue that was used in the sophisticated computer modeling of ultrasonic waves.
In response to this critical medical concern, collaborative research is ongoing to develop this advanced ultrasonic technology. By implementing a real-time technique to determine the pathology of surgical margins during breast cancer surgery, the Utah team seeks to alleviate unnecessary patient suffering and reduce health care costs for those who are diagnosed with breast cancer.
click here to see animation . Computer simulation of scattered ultrasonic waves. The elliptical structures model cross-sections through simulated breast gland ducts. Simulated adipocytes (fat cells) are represented by the larger orange cells.
click here to see animation. Total ultrasonic wave field, showing the incident wave plus the scattered wave.