Kullervo Hynynen, PhD – khynynen@sri.utoronto.ca
Alison Burgess, PhD
Yuexi Huang, PhD
Physical Sciences Platform, Sunnybrook Research Institute
Department of Medical Biophysics, University of Toronto
Toronto, ON Canada
William Querbes, PhD
Dinah W. Sah, PhD
Alnylam Pharmaceuticals Inc,
Cambridge, MA, USA
Popular Version of Paper 3aBAa3
Presented Wednesday morning, June 5, 2013
ICA 2013 Montreal
Huntington’s disease (HD) is an inherited brain disorder which affects motor coordination, and leads to cognitive decline and neuropsychiatric problems (1). The disease is caused by a mutant gene which has a 50% chance of being passed on from an affected parent to a child. Although the disease has been well characterized, no treatment exists (2).
Gene therapy would represent the ideal therapeutic approach for HD. Researchers have demonstrated gene therapy agents can reduce Huntingtin gene expression and lead to significant improvements in symptoms (3,4). However, due to the presence of the blood-brain barrier (BBB), gene therapy agents given orally or intravenously are not able to get into the brain and therefore are not effective. Currently, gene therapy is only effective when delivered directly to the brain using surgical methods.
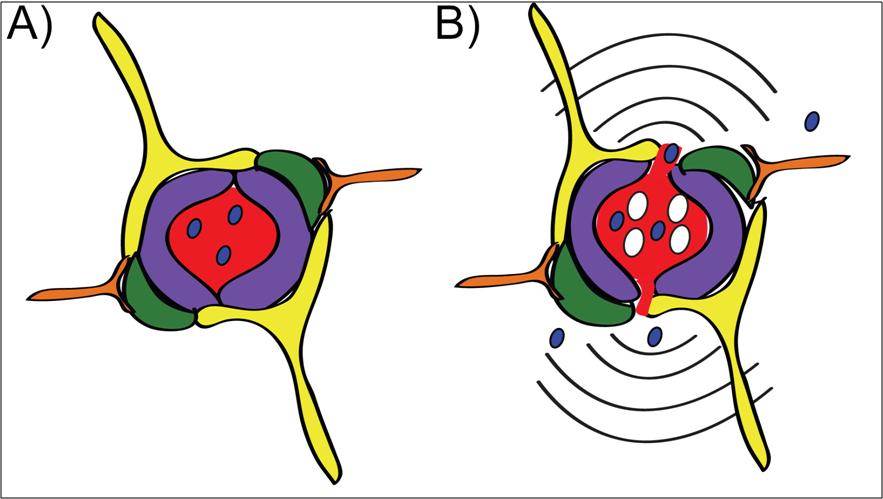
Focused ultrasound is an emerging technology which temporarily disrupts the BBB in a specific brain region to allow passage of molecules, including drugs, into the brain where they are needed most. Therapeutic focused ultrasound works in combination with intravenously delivered microbubbles which are a common imaging agent. When the small circulating microbubbles pass through the focal spot of the ultrasound, the microbubbles expand and contract, stretching the blood vessel walls and leading to temporary BBB disruption (Figure 1).

Figure 1: A) In a brain blood vessel, drug (blue) cannot leave the vessel and enter the brain tissue because of the blood-brain barrier (BBB). B) Focused ultrasound combined with microbubbles (white) temporarily disrupts the BBB and allows drug to flow into the brain for treatment of disease.
In this study, we developed small interfering RNAs (siRNAs) which were designed to interfere with the expression of the Huntingtin gene. MRI was used to identify the target region (striatum) and guide the focused ultrasound treatment (Figure 2a). The siRNAs were delivered intravenously at the onset of the ultrasound treatment and Huntingtin expression was measured 48 hours later.
Using MRI, we confirmed that the BBB disruption was confined to the striatum in the right hemisphere (Figure 2b) indicating that siRNA can only be delivered to brain regions where it is required thereby limiting potential side effects. The analysis of Huntingtin expression demonstrated that the siRNA caused a significant reduction in expression after 48 hours (Figure 3a). The extent of BBB disruption ranged from 12-50% and was positively correlated with the reduction of Huntingtin (Figure 3b). Overall, we observed an average decrease of 32%, similar to what has been achieved in other studies.
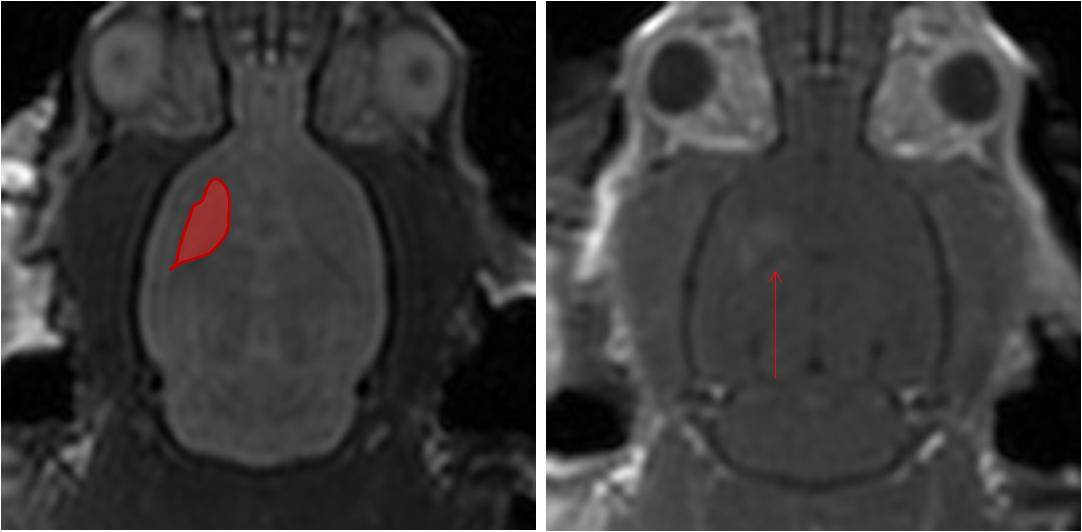
Figure 2: A) MRI is used to identify the target brain structure and guide the focused ultrasound beam for treatment. B) After treatment, MRI is also used to confirm BBB disruption only occurred in the area of interest (bright spots indicated by red arrow).
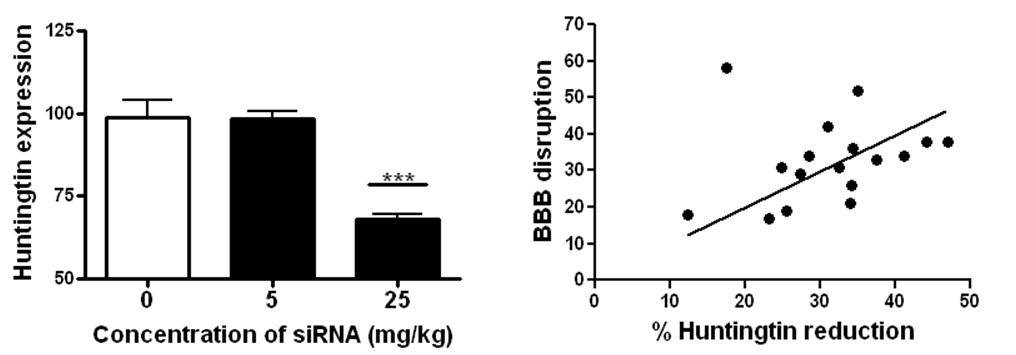
Figure 3: A) The expression of Huntintin was unaffected in by low doses of siRNA. At 25 mg/kg, sufficient siRNA was able to get into the brain with focused ultrasound to significantly reduce Huntingtin expression. B) The amount of BBB disruption was correlated to the percent reduction of Huntingtin expression.
There are clear advantages for using focused ultrasound to deliver siRNA to the brain of Huntington’s patients. First, a major limitation in HD research has been adequate distribution of drug to brain areas in sufficient quantities to observe an effect (5). We have clearly demonstrated that sufficient quantities of siRNA can be delivered to the striatum to cause a significant reduction in Huntingtin expression. Second, the effects of siRNA in vivo can persist for approximately 1 month; however, treatment for HD will require intervention over many years. Focused ultrasound provides a unique treatment option in this regard as the therapy is non-invasive and can be safely repeated multiple times (6).
This study indicates that siRNA delivery to the brain using focused ultrasound represents a promising therapeutic approach that warrants further investigation for the treatment of HD. Ongoing studies using mouse models of HD will determine the impact of a 32% reduction in Huntingtin expression on the symptoms of HD.
References: