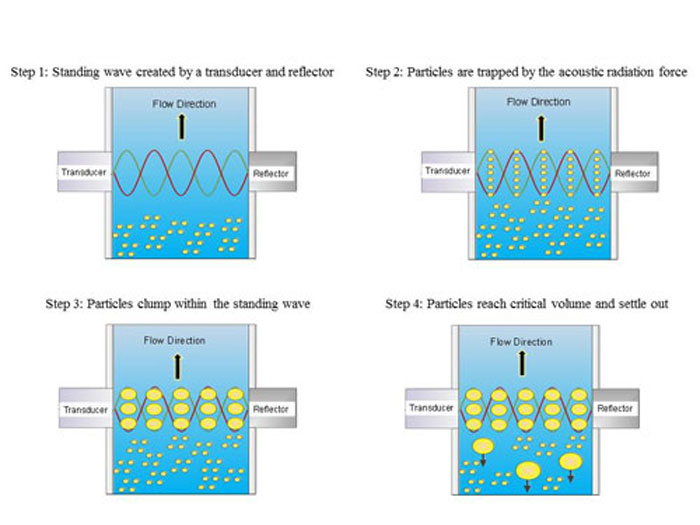
Figure 1. Schematic illustrating process of using ultrasonic standing waves for filtration of suspended particles that are heavier than water, such as yeast particles of mammalian cells.
Brian McCarthy – b.mccarthy@fdsonics.com
Ben Ross-Johnsrud – b.johnsrud@fdsonics.com
Bart Lipkens
FloDesign Sonics, Inc.
380 Main Street
Wilbraham, Massachusetts, 01095
Bart Lipkens – blipkens@wne.edu
Department of Mechanical Engineering, Western New England University
1215 Wilbraham Road, Box S-5024
Springfield, Massachusetts, 01119
Popular Version of Paper 4aPA3
Presented Thursday Morning, May 8, 2014
167th ASA Meeting, Providence
---------------------------------
Cell processing occurs in many technologies such as blood management and transfusion, biopharmaceutical manufacturing, and the food and beverage industry. Currently, centrifuges and filters are used in the preprocessing and filtration stages. These technologies suffer from several drawbacks; filters experience clogging and fouling, and centrifuges do not operate continuously, need expensive cleaning and sterilization, and are difficult to transform into single use concepts, i.e., disposable designs. Continuous cell filtration using ultrasonic standing waves offer an attractive alternative with few of the drawbacks of traditional filtration. Advantages of ultrasonic particle filtration are continuous operation with no mechanical moving parts, no risk of membrane fouling, and no consumables. In biopharmaceutical manufacturing, mammalian cell and bacterial fermentation broths are used for the production of biologics such as recombinant therapeutic proteins, monoclonal antibodies, and vaccines. The clarification of these biological products requires a process that ensures high yield, product consistency, and reproducibility. However, significant variability exists in cell culture turbidity (cloudiness) and viability (the percentage of living cells with respect to the total number of living and dead cells). Ultrasonic filtration is effective for primary clarification, i.e., removal of cells and cell debris, across a wide range of cell density (amount of cells per milliliter of fluid, typically on the order of 10 – 30 million cells per ml), turbidity, and viability. Similarly, ultrasonic filtration can be used in other applications such as the primary clarification to remove bulk yeast from fermented beer, separating blood components for blood transfusion, and separating oil from oil contaminated water.
The acoustic resonator is designed to create a high intensity 3-D ultrasonic standing wave that results in an acoustic radiation force (the acoustic force that acts on a suspended particle) that is larger than the combined effects of fluid drag and buoyancy, and is therefore able to trap, i.e., hold stationary, the suspended particles. As more particles are trapped in the standing wave, rapid aggregation or clumping takes place. When the particle clumps reach a critical size, the buoyancy force becomes dominant, and the particle clumps will settle out, when particles are heavier than water, or rise out of suspension when particles are lighter than water. This process is shown in Figure 1.

Figure 1. Schematic illustrating process of using ultrasonic standing waves for filtration of suspended particles that are heavier than water, such as yeast particles of mammalian cells.
Initial testing has been done with a small scale unit that operates at feed flow rates of about two liters per hour. The system comprises a 1” x 1” flow section and is powered by a 2 MHz PZT-8 transducer. The feed flow is the input suspension, the permeate flow is the clarified fluid outlet, and the concentrate flow outlet contains the cells separated from the fluid (see Figure 2). The concentrate flow rate varies from 0.2 to 0.4 liters per hour, depending on the mass concentration of the feed. Permeate flow rates are then from 1.6 to 1.8 liters per hour. Separation efficiencies in excess of 95% are obtained for yeast suspensions with starting mass concentrations ranging from 0.5 to 3%. Testing has also been done with a variety of different CHO (Chinese Hamster Ovary) cell lines. CHO cells are by far the most common mammalian cell used in bio-pharmaceutical production. Measurements indicate that the acoustic field does not affect the viability of the cells nor has any impact on the nature of the expressed protein in the fluid, i.e., thereby represents a benign effect on the cells and the protein. Trapping of CHO cells in the ultrasonic standing wave device is shown in Figure 3. Filtration efficiencies are typically in excess of 90% across a wide range of cell viability, cell density and suspension turbidity.
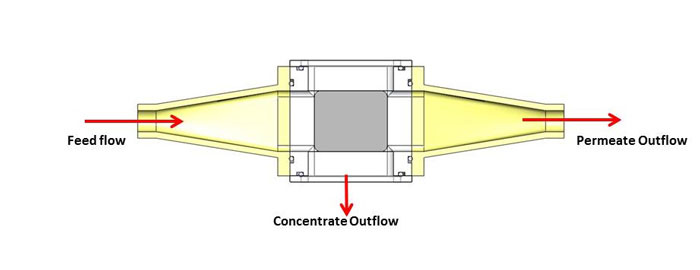
Figure 2. Schematic showing the feed flow, permeate flow, and concentrate flow for an acoustic separation device operating in the horizontal orientation.
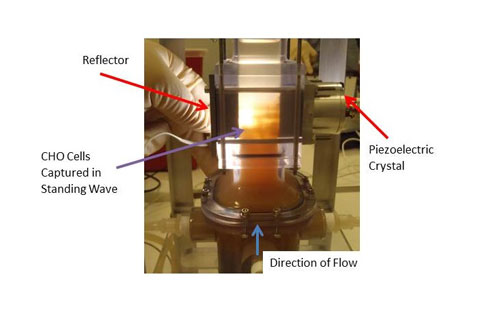
Figure 3. Trapping of CHO cells in the ultrasonic standing wave device. The main flow field is in the vertical upward direction.
Figure 4 shows a photo of the original yeast suspension (3% solids) on the left, the clarified permeate flow in the middle, and the concentrated yeast collection on the right.

Figure 4. Photo of original yeast suspension (3% solids) on the left, clarified fluid in the middle, and concentrated yeast collection on the right for a typical experiment at a feed flow rate of three liters per hour.
A large scale model was built with a ten times larger flow section powered by three PZT-8 transducers operating at the same frequency. Yeast filtration efficiencies in excess of 90% were obtained for flow rates of up to 22 liters per hour. The large scale model shows that it is feasible to achieve flow rates that make the technology attractive to food and beverage industries and bio-pharmaceutical companies for applications of cell clarifications.
A video is attached that shows the acoustic filtration process of yeast in action. The suspension flows from bottom to top. The yeast particles are trapped in the standing wave forming lines of particle clumps. When the clumps reach a critical size, they drop out of suspension, and fall down. This video was taken for a yeast suspension with a 3% mass concentration.