Cyril Lafon - lafon@lyon.inserm.fr
David Melodelima
Jean-Yves Chapelon
Dominique Cathignol
INSERM U556
151 Cours Albert Thomas
69003 Lyon, France
Popular version of paper 2aBB5
Presented Tuesday morning, May 25, 2004
147th ASA Meeting, New York, NY
High Intensity Focused Ultrasound (HIFU) is now an accepted therapeutic method for removing tumors. In this approach, intense ultrasound waves focus inside the body to heat up a tumor and coagulate it. This process of intense heating is known as "thermal ablation" and several devices for carrying out this task are currently marketed. This method of destroying tumors is non-invasive when using ultrasound sources that are "extracorporeal," or originate from outside the body.
However, extracorporeal HIFU is not always suitable for treating some tumors, such as ones that are deeply buried or "deep seated." Moreover, intervening tissue between the ultrasound device and the tumor may contain bones or gaseous pockets, in which the ultrasound wave cannot propagate. While traveling through tissue, an ultrasound wave is naturally attenuated (weakened) and deformed (e.g., phase-aberrated) in structures with different geometric and acoustic properties. Attenuation or phase aberration during treatment of deep-seated tumors results in a decrease in the amount of sound pressure that can be delivered to destroy a tumor. In this case, sound pressure can be increased at the surface of the ultrasound-generating device in the hope of supplying sufficient energy to the point inside the body where the ultrasound focuses (i.e., the tumor). However, this increase is to the detriment of intervening tissue whose temperature will also rise.
Therefore, we have developed a new minimally invasive approach, one that uses a device known as an "interstitial applicator," which brings the ultrasound source into contact with the target via natural routes in order to minimize the effects of attenuation and phase aberration. It then becomes possible to use higher ultrasound frequencies, which increases the absorption of ultrasound in the desired area and thereby leads to a more efficient heating of the treatment region. In contrast to extracorporeal applicators, interstitial probes impose additional design constraints with regard to size and ergonomics.
In this paper, the technological and clinical research carried out by Unit 556 of the French National Institute for Health and Medical Research (INSERM) over the last years will be presented. Our research aims to develop ultrasound endoscopic applicators for treating cancers of the esophagus and the biliary duct. Systemic treatments such as radiotherapy are not effective on these digestive cancers. Surgery is the only curative treatment available but very few patients are able to undergo this surgery because of their general condition and the late diagnosis of these tumors. Prognosis is extremely poor and only palliative care (placing of prostheses) can be undertaken. These tumors develop locally around the lumens (spaces) in the biliary or esophageal organs and are therefore good candidates for local treatment by intraluminal thermotherapy (Figure 1).
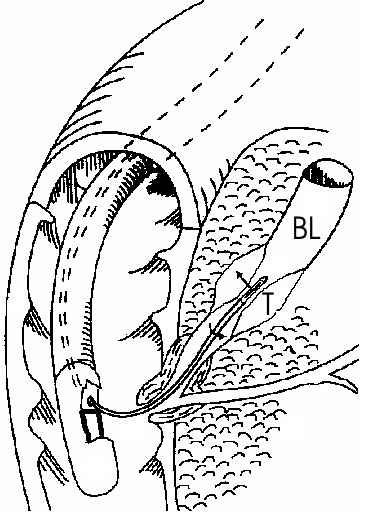
Figure 1. Endoscopic application of high intensity ultrasound on tumors (T)
in the biliary lumen (BL). The applicator is brought into contact with the tumor.
The technique uses non-focalized plane transducers, ultrasound generators that do not focus ultrasound to tiny points but instead produce "plane waves," sound waves whose fronts form flat parallel planes. The transducer destroys tumors through the process of coagulation necrosis, in which heated tumor cells agglomerate into a lifeless mass. To compensate for the absence of focalization, it is necessary to operate the transducer at a high frequency (10 MHz). The treatment depth is directly related to the ultrasound frequency. The higher the frequency, the less the ultrasound penetrates the tissue, and the more intense the heat. Even when coagulating cylindrical volumes of tissue are required for covering the total volume of the tumor, a rotating transducer is preferred to a tubular transducer. Indeed, the divergence of acoustical waves associated with tubular transducers results in a rapid fall in sound pressure that limits the depth and/or increases the duration of treatment. In order to minimize the movement of the applicator, we have developed an ultrasound cylindrical phased array composed of 64 elements for transesophageal thermal ablation. Based on the principal of dynamic focalization, the 64 small transducers mounted on a tubular frame are excited successively. Depending on the delay times of the excitation of each element, a plane wave can be generated with a group of elements (Figure 2). Rotation of the plane wave is obtained by exciting a group of neighboring transducers. Depending on the application, different guiding methods were tested: ultrasound (Figure 3), MRI (Figure 4) and fluoroscopy. The interest of MRI lies in the fact that this imaging modality can give in almost real time temperature monitoring whilst controlling the extent of coagulation necrosis. However, it requires constructing non-magnetic applicators that do not induce artifacts on the image.
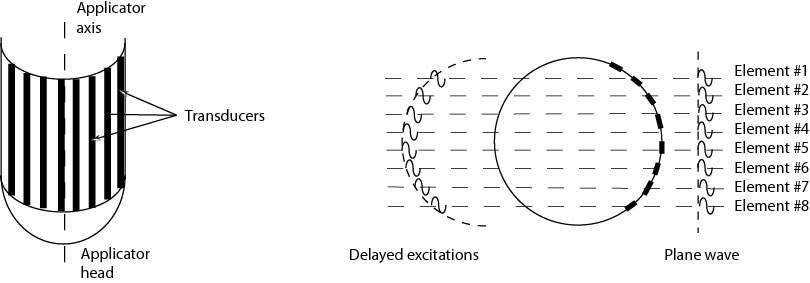
Figure 2. Principle of generation of a plane wave from a cylindrical array.
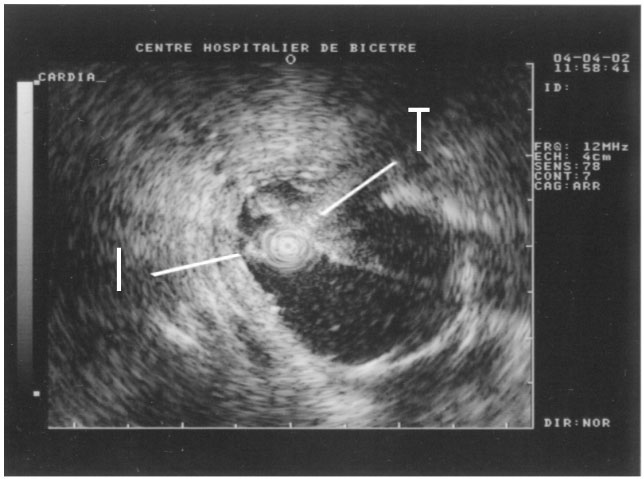
Figure 3. Ultrasound scan in the esophagus. The ultrasound miniprobe (I)
is positioned in front of the transducer (T) of the therapeutic applicator.
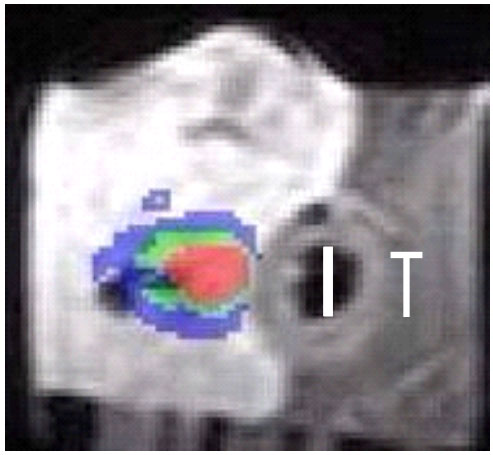
Figure 4. Temperature map measured by MRI in vitro in liver in a plane perpendicular
to the heating transducer (T). Temperatures are color-coded : blue between 47
and 57°C, green 58 - 67°C and red above 67°C.
A pilot study was carried out in 10 patients who were diagnosed with cholangiocarcinoma, malignant tumors in the biliary duct system. The applicator used was compatible with conventional gastrointestinal endoscopic equipment. A guide wire enabled the applicator to exit the endoscope and move up into the biliary ducts to the tumor. The method is minimally invasive since the duration of anesthesia was not significantly prolonged and both the treatment and the control examinations corresponded to the dates when prostheses (such as stents which keep the biliary duct open) needed to be renewed. For some patients, who underwent surgery after ultrasound treatment, analysis of the removed tumor demonstrated 10 mm deep coagulation necrosis at the targeted point. In most cases, local tumor regression was observed with proliferation in the distal end (farther from the center of the body) resulting from a pre-treatment under-estimate of tumor spread. In one case, the tumor was completely destroyed and bile flow restored (Figure 5). Future studies should determine whether this endoscopic ultrasound treatment of biliary tumors is a new palliative method (one that relieves symptoms but does not eliminate the disease) or whether it could be curative in high-risk surgery patients exhibiting localized tumors.
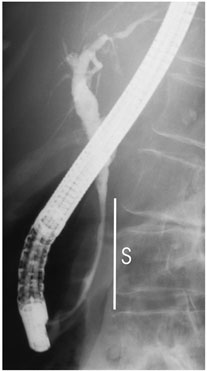
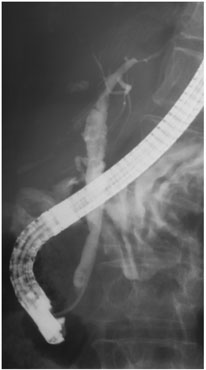
Figure 5. On this patient, the stricture (S) in the bile duct was pronounced
before treatment by fluoroscopy (a)(left). It disappeared after intraductal
treatment by high intensity ultrasound (b)(right).
Conclusion:
When used with modern imaging modalities, interstitial therapeutic ultrasound
devices offer very promising options for cancer treatment. These methods are
suitable for treating deep-seated tumors, and are precise, safe, repeatable,
bloodless and economic.