Targeted Ultrasound Contrast Agents for the Imaging of Biofilm Infections
Pavlos Anastasiadis pavlos@hawaii.edu
Dept. of Mech. Eng.,
Univ. of Hawaii at Manoa
2540 Dole St.
Honolulu, HI 96822
Kristina Mojica
Univ. of Hawaii at Manoa,
Honolulu, HI 96822
Michelle L. Matter
Univ. of Hawaii at Manoa,
Honolulu, HI 96822
John S. Allen
Univ. of Hawaii at Manoa,
Honolulu, HI 96822
Popular version of paper 2aBB10
Presented Tuesday Morning, May 19
157th ASA Meeting, Portland, OR
Introduction
A biofilm can be briefly described as an aggregate of microorganisms imbedded in a gel-like matrix that is composed of microbially produced substances, the exopolymeric substances, and attached to a surface. Biofilms can be regarded as highly organized structures of microorganisms whose importance to health care and diseases continues to be recognized. The three-dimensional heterogeneous assemblages of microbial cells show similar characteristics to a matrix-like structure where the exopolymeric substances provide bacteria with a protective degree of stability, a primitive circulatory system and a framework for the development of cooperative and specialized community functions.
Opportunistic pathogenic bacteria can invade the human circulatory system. The intruding microorganisms may attach to surfaces within the body and form multilayered cell clusters followed by intercellular adhesion that leads to the development of confluent bacterial biofilms. The microorganisms that are most frequently associated with biofilm infection are Viridians streptococci, Staphyloccocus aureus, Enterococcus faecium, Staphyloccocus epidermidis and Pseudomonas aeruginosa. Biofilm infections such as endocarditis (biofilm infection of the heart valves) are particularly difficult to treat due to the biofilm’s resistance to antimicrobial and antibiotic agents. Restricted penetration, decreased growth rate and the expression of resistance genes have been proposed as potential explanations, but the underlying resistance mechanisms are still not well understood. Serious complications of bacterial biofilms include life-threatening infective endocarditis and alongside increased risk of myocardial infarction or stroke. The incidence of infective endocarditis is approximately 1.7 – 6.2 cases per 100,000 patients per year while mortality approaches 40%. Endocarditis may originate from the infection of a prosthetic heart valve; moreover, a plethora of indwelling medical devices such as replacement joints, venous catheters, and cerebrospinal fluid shuts are prone to biofilm formation. Colonization leads to blockages, and loss of function of the device. In particular, Staphyloccocus aureus followed by Pseudomonas aeruginosa are mainly associated with implanted medical devices such as prosthetic heart valves and artificial limbs. Moreover, biofilm infected endoscope tubing has been estimated by the U.S. Centers for Disease Control and Prevention to be a source of transmission of 270,000 infections per year. Finally, there is growing evidence that biofilms play an important role in a many ocular infections infecting materials such as scleral buckles and contact lens.
Detection and Imaging of Biofilms
In order to face the growing challenge of biofilm-related diseases, a better understanding of the fundamentals of the biofilm composition is needed. The main elements responsible for the morphology and function of biofilms are the exopolymeric substances (EPS), and thus, are considered to be the key components that determine the physicochemical and biological properties of biofilms. The presence of EPS can be investigated by chemical methods or microscopic techniques. Biochemical analysis involves the isolation of EPS by extraction procedures, which result in the disruption of the biofilm matrix while microscopy techniques are used for measuring the chemical properties of biofilms with fluorescent markers that act toxically to biofilms.
Conventional biofilm detection and imaging methods typically require destructive autopsies of both the biofilm and substrates. The most prevalent among the traditional methods are microscopy and confocal scanning laser microscopy (CSLM). CSLM is the method most frequently employed for 3D biofilm imaging. Nonetheless, a significant limitation is that these techniques cannot be directly used in clinical setting for the in vivo examination of patients. Likewise, these techniques cannot be used to visualize unstained material or any other matrix which was not specifically treated. In fact, currently there is no established method for detecting and imaging biofilms in situ and in vivo, and thus their presence often goes undetected until the later stages of the disease when the chances of successful intervention can be quite limited. Endocarditis, for instance, is typically only seen with conventional diagnostic ultrasound when the growth of the biofilm on the heart valve, known as vegetation, impedes the underlying blood flow. A better understanding of the development processes needs to be achieved as well as an improved means for early detection. A robust molecular imaging technique would be a significant advancement in the diagnosis and treatment of biofilm infections.
Ultrasound offers advantages over other modalities in terms of relative low-cost and its ability to be manufactured in a portable size. Advances in high-frequency transducers have made it possible to achieve greater resolution for distinguishing the underlying characteristics of diseased tissue and could further assist and enhance in the imaging of biofilm structures. Ideally, a non-invasive, nondestructive method that would allow in situ and in vivo evaluation of biofilm characteristics, would greatly expedite translational research efforts for potential clinical applications. High-frequency scanning acoustic microscopy (SAM), in particular, offers a novel means to obtain structural and mechanical insights of living biofilms as it operates well below the damage threshold of biofilm materials. SAM takes advantage of the elastic response of the material to acoustic waves and, therefore, provides information on local changes in mechanical properties without any physical contact with the specimen. Acoustic microscopy enables the real-time and non-invasive monitoring of physiological processes in living samples (Figure 1).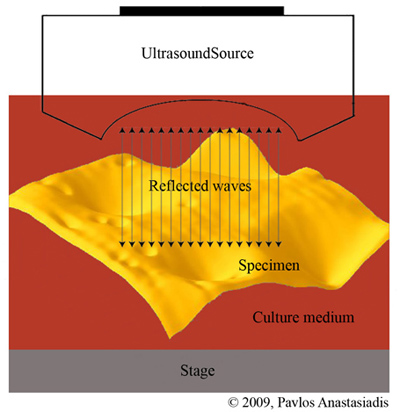 |
| Figure 1. Scanning acoustic microscopy. The ultrasound waves are reflected back from the biofilm surface and used to form an image. |
Ultrasound contrast agents are encapsulated microbubbles with a polymer, protein or lipid shell. Small proteins can be attached onto the surface of microbubbles to target specific sites of interest within the body for diagnostic or therapeutic purposes. A common way to achieve this is to exploit the highly specific interaction of avidin, as found in egg albumen, with biotin (vitamin H). The affinity of avidin for biotin is the strongest known non-covalent -- a type of chemical bond -- interaction of a protein and ligand and allows biotin-containing molecules in a complex mixture to be discretely bound with avidin conjugates. Since biotin is a relatively small molecule, it can be attached to a number of proteins without significantly altering their biological behavior. The biotin-avidin bond acts as a bridge that allows attaching a variety of useful antibodies onto a microbubble’s surface. The resulting targeted microbubbles thus can be developed and recently been shown to be effective tools for the molecular imaging of cancer, thrombosis and atherosclerotic plaque. One important characteristic of microbubbles is that they strongly scatter acoustic waves and provide unique acoustic signals. This effect is enhanced as sufficient acoustics pressure amplitudes at which they oscillate non-linearly about their equilibrium radius at sufficient acoustic forcing. For targeted ultrasound contrast agents (Figure 2), they add molecular imaging capabilities to ultrasound, facilitating the differentiation of diseased from healthy tissue in ultrasound images.
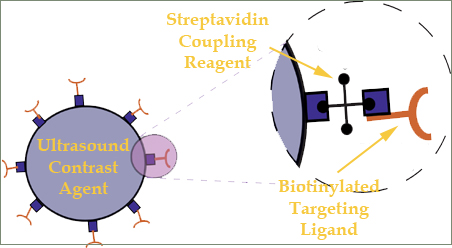 |
| Figure 2. Targeted contrast agents are conjugated with antibodies which remain attached on their surface as they bind to specific diseased sites. |
Summary and Future Directions
The current study encompasses a novel method to image biofilm formation and structure. We investigated in vivo bacterial biofilms of Staphyloccocus aureus and Pseudomonas aeruginosa with high frequency ultrasound and targeted ultrasound contrast agents. This work investigates the binding ability of targeted ultrasound contrast agents to the biofilm matrix. Lipid-encapsulated microbubble contrast agents with a mean diameter of 2.5 micrometers were used in combination with high-frequency acoustic microscopy. Although various successful attempts to quantify biofilm structure have been made, producing a clear image of the biofilm or obtaining reliable data about its thickness, density or biomass still presents a challenge. Some of these outstanding issues can be addressed with acoustic microscopy.
For this investigation, lectins have been employed as probes for the targeting of the biofilm matrix during its growth process. Lectins are proteins of plant, animal or microbial origin, which bind to the carbohydrates of the extracellular matrix of the biofilms and likewise are used in the study. The use of fluorescently labeled lectins allowed the microscopical in situ detection of EPS and the 3D surface representation of the Staphyloccocus aureus biofilms (Figure 3). In this figure, the targeted microbubbles conjugated with lectins are shown in red while the matrix appears green over a period of 96 hours of biofilm growth. The matrix increases in mass in conjunction with an increase binding rate of targeted agents. Knowledge of the 3D structure of a biofilm is important in order to understand its unique biochemical and mechanical characteristics as well as to monitor the efficacy of procedures to treat or eliminate it.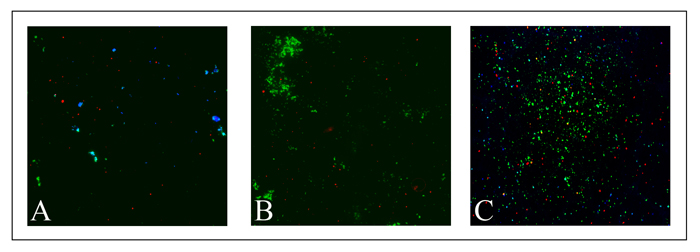 |
| Figure 3. Growth of Staphyloccocus aureus biofilm matrix at time points of (A) 24 hours, (B) 72 hours and (C) 96 hours. Blue shows the microbial cells, red the targeted microbubbles and the matrix appears in green. The increase in matrix mass growth is noticeable over the time course. |
Targeted microbubbles in particular offer the potential to provide new insights at the molecular level and to further assist understanding the complexity of biofilm behavior. In addition the targeted agents allow for ultrasound detection. This is highlighted in figure 4 which shows an image obtained by a high-frequency acoustic microscope (100 MHz). The bright yellow spots show the bound agents. The brightness is related to the increase in acoustic backscatter compared to the biofilm matrix. The associated acoustic signature is specific and localized to the biofilm sites allowing the molecular imaging of the infection within healthy tissue areas.
Targeted ultrasound contrast agents offer a potential means in which biofilms infections could be imaged for in vivo clinical applications. The study shows the enhanced backscatter from the agents at high frequency which is particularly relevant to ocular, dermal and intra-vascular applications where high-frequency transducers are utilized. Diagnosis of catheter infections might be monitored with the injection of targeted agents and a high frequency probe. Investigation of targeted agents at lower conventional diagnostic frequencies is currently in progress in which the nonlinear acoustic characteristics of the contrast agents can be exploited. Refinement of the technique with specific ligands may allow for the determination of the specific infecting bacterial species. Future work also will examine the potential for the use of ultrasound contrast agents as a therapeutic treatment for biofilm infections. Thus, acoustically ruptured agents could be used to penetrate biofilm structures and facilitate the delivery of antimicrobial or antibiotic drug agents.
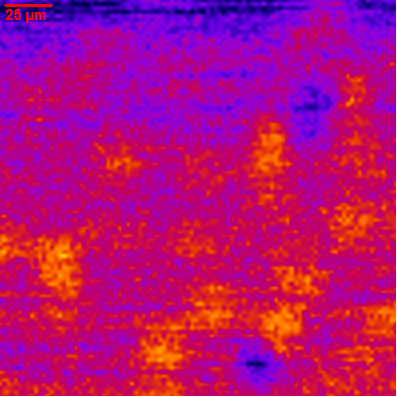 |
| Figure 4. High-frequency acoustic microscopy image of a biofilm matrix with bound targeted agents. The bright yellow spots indicate the location of the bound targeted contrast agents. The relative acoustic backscatter scales with the intensity of the color map. |